M Rna Vaccines And Therapeutics Regulatory Landscape
Regulatory Landscape - Overview
mRNA Vaccines And Therapeutics Regulatory Landscape: Product Overview
mRNA vaccines and therapeutics are class of medical treatments that utilize synthetic messenger RNA (mRNA) to instruct the body’s cells to produce specific proteins. In the case of vaccines, these proteins mimic parts of a virus or pathogen, making the immune system to recognize and respond to future infections. For therapeutics, the mRNA directs cells to produce Specific proteins, which help to treat or manage diseases.
mRNA Vaccines And Therapeutics Applications:
mRNA vaccines offer rapid development and broad protection against various infectious diseases such as influenza, RSV, Zika, rabies, Ebola, and COVID-19 variants. They also support innovative delivery systems that enhance mucosal immunity, making them effective tool in modern disease prevention.
One of the most promising uses of mRNA technology is in oncology. Personalized cancer vaccines are being developed to encode tumor-specific antigens, enabling the immune system to recognize and attack cancer cells.
mRNA is showing promise in treating rare inherited diseases such as methylmalonic acidemia and glycogen storage disorders. These conditions often lack effective treatments, and mRNA offers a less invasive and more adaptable alternative to traditional gene therapy.
Researchers are investigating how mRNA can be used to modulate immune responses in autoimmune diseases like multiple sclerosis and rheumatoid arthritis. By fine-tuning immune activity, mRNA could help reduce inflammation and prevent immune attacks on healthy tissue.
mRNA Vaccines And Therapeutics Product Development steps:
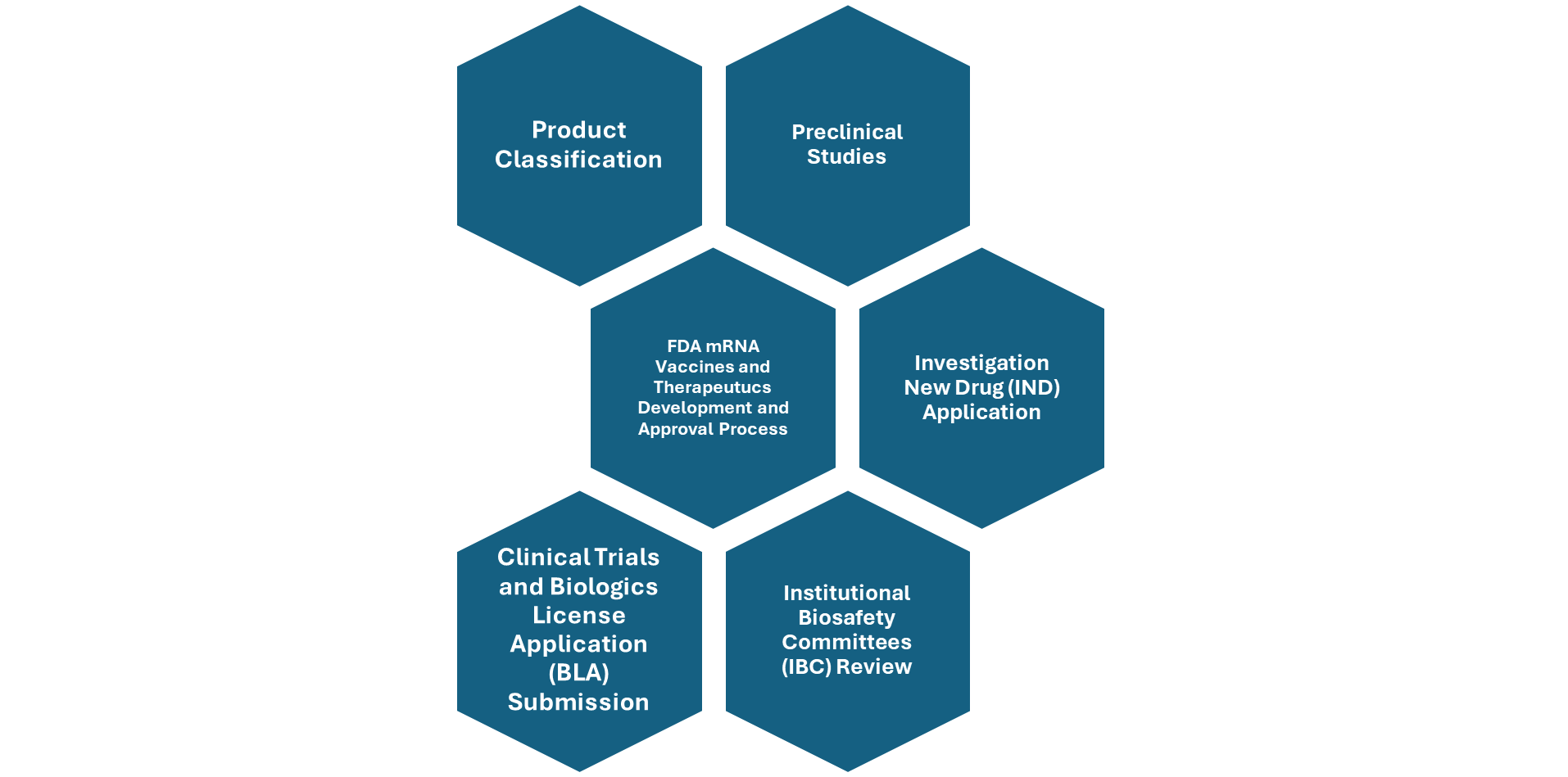
Figure: overview of FDA mRNA Vaccines and Therapeutics Approval and Development Process
mRNA Vaccines And Therapeutics Market Size Overview:
mRNA Vaccines and Therapeutics Market Size was estimated at 6.06 (USD Billion) in 2023. The mRNA Vaccines and Therapeutics Market Industry is expected to grow from 7.23 (USD Billion) in 2024 to 50.0 (USD Billion) by 2035. The mRNA Vaccines and Therapeutics Market CAGR (growth rate) is expected to be around 19.22% during the forecast period (2025-2035).
mRNA Vaccines And Therapeutics Regulatory Landscape:
There are several key regulatory agencies who oversee the approval and monitoring of mRNA Vaccines And Therapeutics to ensure their safety, efficacy, and quality.
|
Regulatory agencies |
Regulatory Ministry |
|
Federal Food and Drug Administration |
United States: Department of Health and Human Services (HHS) |
|
The Medicines and Healthcare products Regulatory Agency |
United Kingdom: The Medicines and Healthcare products Regulatory Agency (MHRA) under the Department of Health and Social Care (DHSC) |
|
Central Drug Standard Control Organization |
India: The Ministry of Health and Family Welfare |
|
South African Health Products Regulatory Authority (SAHPRA) |
National Department of Health. |
|
Pharmaceuticals and Medical Devices Agency (PMDA) |
Japan: Ministry of Health, Labour and Welfare. |
|
National Medical Products Administration (NMPA) |
China: The Ministry of Health |
|
Health Sciences Authority |
Singapore: The Ministry of Health |
|
European Medicine Agency |
European union |
|
Brazilian Health Regulatory Agency (Anvisa) |
Ministry of Health, part of the Brazilian National Health System (SUS) |
mRNA Vaccines And Therapeutics Guidelines:
mRNA-based therapies are emerging as a transformative approach for treating a wide range of diseases that are resistant to conventional treatments, including infectious diseases, metabolic genetic disorders, cancer, cardiovascular, and cerebrovascular conditions. These therapies offer several advantages, such as high efficacy, minimal side effects, and ease of production.
The success of mRNA vaccines during the COVID-19 pandemic, particularly BNT162b2 by Pfizer-BioNTech and mRNA-1273 by Moderna, demonstrated the potential of this technology. These vaccines showed approximately 90% effectiveness in preventing infection in fully vaccinated individuals and around 80% in those partially vaccinated.
mRNA Vaccines And Therapeutics Classification of the Product:
mRNA Vaccines And Therapeutics Regulatory Process Overview, By Country:
mRNA products are classified as biologics by the U.S. Food and Drug Administration (FDA), The Center for Biologics Evaluation and Research (CBER) under Food and Drug Administration (FDA) is responsible for the regulation of all the biologics including mRNA vaccines and therapeutics and make sure that several important regulatory steps must be followed to ensure safety, efficacy, and compliance.
Regulatory framework requires that, sponsors must submit an Investigational New Drug (IND) application to the FDA’s Center for Biologics Evaluation and Research (CBER).
This application must be approved and assigned a biologics IND number before any human trials can begin. Given that mRNA involves genetic material, biosafety reviews by Institutional Biosafety Committees (IBCs) may also be required, particularly when synthetic or recombinant mRNA is used.
In different regions, the oversight bodies responsible for reviewing the ethical aspects of clinical trials and biomedical research have different names:
- In Canada, this body is known as a Research Ethics Board (REB).
- In the European Union (EU), it is referred to as an Ethics Committee (EC).
- In Japan, the equivalent is called an Ethical Review Committee (ERC).
These committees serve a similar function: ensuring that research involving human participants is conducted ethically, with proper informed consent, risk minimization, and participant protection. Additionally, because mRNA is typically delivered using lipid nanoparticles (LNPs), the safety and composition of these delivery systems are also subject to regulatory review.
If human clinical trials demonstrate positive outcomes, sponsors can proceed to seek full regulatory approval for their mRNA-based product by submitting a Biologics License Application (BLA) to the Center for Biologics Evaluation and Research (CBER).
In response to the urgent public health threat posed by the COVID-19 pandemic, the mRNA-based vaccines developed by Pfizer/BioNTech and Moderna were initially granted Emergency Use Authorizations (EUAs) to allow for rapid deployment. As more comprehensive data on their safety and efficacy became available, both vaccines later received full regulatory approval through Biologics License Applications (BLAs), marking their transition from emergency use to standard authorization.
The manufacturing process must comply with Good Manufacturing Practices (GMP) to ensure product quality and consistency. Before initiating clinical trials, sponsors must provide detailed preclinical data, including toxicology studies, to demonstrate safety and support the IND submission.
Once trials are underway, there is a requirement for ongoing safety monitoring, submission of annual reports, and prompt reporting of any adverse events to the FDA. These steps collectively ensure that mRNA-based therapies are developed responsibly and safely.
mRNA Vaccines And Therapeutics updates:
April 2025, Arcturus Therapeutics has received Fast Track Designation from the U.S. FDA for its self-amplifying mRNA (sa-mRNA) vaccine candidate, ARCT-2304, also known as LUNAR-H5N1. This vaccine is designed to protect against pandemic influenza A virus H5N1, a virus with significant global health risk potential.
The Fast Track status is intended to accelerate the development and review of drugs and vaccines that address serious conditions and unmet medical needs. This designation provides benefits such as enhanced communication with the FDA, eligibility for priority review, and rolling submission of data.
January 2023, Under the “Mission COVID Suraksha”, the Government of India successfully supported the development of four indigenous COVID-19 vaccines within two years, showcasing the country’s scientific and manufacturing capabilities. These vaccines include ZyCoV-D, the world’s first DNA-based COVID-19 vaccine; CORBEVAX™, India’s first protein subunit vaccine; GEMCOVAC™-19, the first indigenously developed mRNA vaccine; and iNCOVACC, the world’s first intranasal COVID-19 vaccine. The initiative was spearheaded by the Department of Biotechnology (DBT) under the Ministry of Science & Technology, with financial backing of ₹900 crore through the Atmanirbhar Bharat 3.0 package. In addition to vaccine development, the mission also facilitated the scale-up of Covaxin production at Bharat Biotech and Indian Immunologicals Limited, reinforcing India’s preparedness for future pandemics through self-reliant, safe, and affordable vaccine solutions.
mRNA Vaccines And Therapeutics Regulatory Challenges and possible risk in development:
One major challenge regarding mRNA therapeutics is to maintain their stability, as they are susceptible to enzymatic degradation by RNases which can compromise their therapeutic efficacy if not properly stabilised.
To prevent degradation use of protective delivery system such as lipid nanoparticles can further complicate the regulatory approval process as this material should be complying in terms of safety, reproducibility and biocompatibility and all other strict regulatory standards.
Moreover, storage conditions for mRNA-based products are particularly demanding, they often require ultra-low temperature which pose significant logistical and distribution challenges especially in low resource settings.
Additionally, immunogenicity presents another hurdle as exogenous mRNA and its delivery agents can trigger immune unintended response which can lead to adverse effects on patients, conducting extensive preclinical toxicology and immunogenicity study is most important to ensure safety.
Lack of harmonization, different countries follow different regulatory framework, making it difficult for the manufacturers to develop their product which will show compliance with most of the countries regulatory framework and can market their product in these countries.
mRNA Vaccines And Therapeutics Competitive Landscape Dashboard:
Companies With Marketed mRNA Vaccines And Therapeutics:
- Arcturus Therapeutics
- Pfizer
- Moderna
- Sanofi
- Johnson and Johnson
- Eli Lilly
- Ginkgo Bioworks
- Regeneron Pharmaceuticals
- Merck
- AstraZeneca
- Translate Bio
- BioNTech
- CureVac
- Genentech
- Novartis
Regulatory Landscape - Table of Content
Table of contents will appear here once available.
Customer Stories

“This is really good guys. Excellent work on a tight deadline. I will continue to use you going forward and recommend you to others. Nice job”

“Thanks. It’s been a pleasure working with you, please use me as reference with any other Intel employees.”

“Thanks for sending the report it gives us a good global view of the Betaïne market.”

“Thank you, this will be very helpful for OQS.”

“We found the report very insightful! we found your research firm very helpful. I'm sending this email to secure our future business.”

“I am very pleased with how market segments have been defined in a relevant way for my purposes (such as "Portable Freezers & refrigerators" and "last-mile"). In general the report is well structured. Thanks very much for your efforts.”

“I have been reading the first document or the study, ,the Global HVAC and FP market report 2021 till 2026. Must say, good info! I have not gone in depth at all parts, but got a good indication of the data inside!”

“We got the report in time, we really thank you for your support in this process. I also thank to all of your team as they did a great job.”