
Regulatory Landscape - Overview
Foley Catheters Regulatory Landscape: Product Overview
Foley catheter is a flexible, hollow tube inserted in bladder via urethra to drain the urine, outside body into a collection bag, usually used for the patient who can’t pee on their own due to various medical conditions. It is a type of indwelling catheter, meaning one that is kept in place so that urine is continuously drained from the bladder.
Types of Foley Catheters
Foley catheters are segmented into two types:
One way catheter- Urinary catheters have 2 ports(lumens) at the distal ends, one of them has funnel shape which allow urine efflux, other is involved in influx of water into retention balloon, is inflation or deflation channel.
Three-way catheter-3-way catheters have one more additional channel. Which is involved in facilitating bladder irrigation or it is used for installation of medication, this is mostly used in urological surgery or in case of blader bleeding or prostate tumor, where bladder needs irrigation in continuous or intermittent form for clearing blood clots or any debris.
Based on the material used they are further segmented into following types:
Latex Foley Catheters: These kind of catheters are flexible and mostly used for short-term medical conditions.
Silicone Foley Catheters: These catheters are suitable for the for patients having latex allergies and can be utilised long-term.
Hydrogel-Coated Catheters: These catheters are said to provide additional comfort, reducing the risk of urethral irritation.
Foley Catheters Applications:
Foley catheters have applications in following medical conditions:
- People anesthetized during surgery, when patient’s bladder control is affected.
- To reduce risk of postoperative urinary retention, catheters are used during or after urethral or bladder surgery.
- People suffering from acute or chronic urinary retention due to enlarged prostate, pelvic organ prolapse, kidney stones, genital injury, or any other medical issues.
- People who are paralysed and can’t control their bladders.
- Patients suffering with kidney disease and requires measuring urine output regularly.
- For women’s who underwent Cesarean section, preventing bladder injury and urinary retention post operation.
- People who are suffering with serious illness and are at end of life, specially the old, aged patients who can’t move or take care of themselves.
- Women with Induced pregnancy, when balloon is inflated behind the cervix to widen it.
- To empty the bladder during childbirth to administer chemotherapy to irrigate the bladder.
Foley Catheter Product Development Steps:
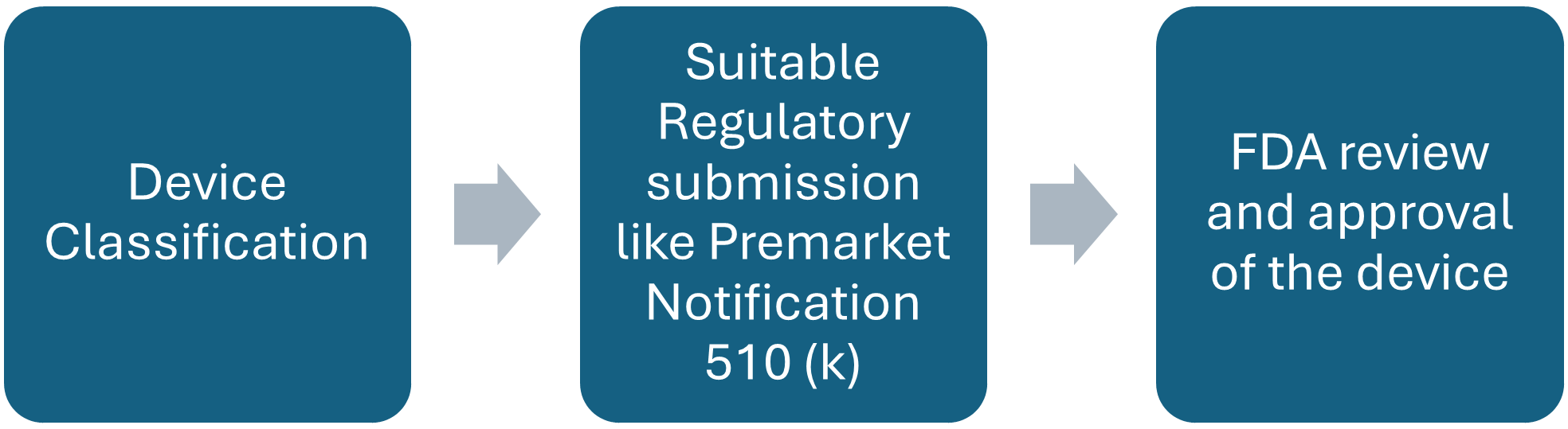
Figure: overview of product development and approval process by FDA.
Foley Catheters Market Size Overview:
Foley Catheters Market was valued at USD 1.7 billion in 2023 and is projected to grow from USD 1.8 billion in 2024 to USD 2.97 billion by 2032, exhibiting a CAGR of 5.30% during the forecast period (2024 - 2032). A big therapeutic portfolio, new product launches, and widespread acceptance of urologic disorders, which are becoming more common, these are the key market drivers enhancing the market growth.
Foley Catheter Regulatory Landscape:
There are several key regulatory agencies who oversee the approval and monitoring of Foley Catheter to ensure their safety, efficacy, and quality.
|
Regulatory agencies |
Regulatory Ministry |
|
Federal Food and Drug Administration |
United States: Department of Health and Human Services (HHS) |
|
The Medicines and Healthcare products Regulatory Agency |
United Kingdom: The Medicines and Healthcare products Regulatory Agency (MHRA) under the Department of Health and Social Care (DHSC) |
|
Central Drug Standard Control Organization |
India: The Ministry of Health and Family Welfare |
|
South African Health Products Regulatory Authority (SAHPRA) |
National Department of Health. |
|
Pharmaceuticals and Medical Devices Agency (PMDA) |
Japan: Ministry of Health, Labour and Welfare. |
|
National Medical Products Administration (NMPA) |
China: The Ministry of Health |
|
Health Sciences Authority |
Singapore: The Ministry of Health |
|
European Medicine Agency |
European union |
|
Brazilian Health Regulatory Agency (Anvisa) |
Ministry of Health, part of the Brazilian National Health System (SUS) |
Foley Catheters Guidelines:
Eligibility: Foley catheters are used mostly during or post-surgery, can also be used for the patients with severe urinary retention, or urinary incontinence cases, not possible to control by other means. This kind of catheters can be used for many days or even weeks, but it is recommended to use only if it is highly required and not for long period of time, as it always pose risk of urinary tract infection, therefore it must be removed as soon as possible, once it is no longer needed.
If other options are available to manage the incontinence or urinary retention, this catheters should not be used as a first option in the treatment, as in severe cases catheters associated UTIs can cause urosepsis, which involves spreading infection into bloodstream, causing deadly immune reaction.
As per centres of disease control and prevention, there is a risk of 3 to 7 % everyday of bacteriuria (increase of bacteria in urine) due to the use of indwelling catheters. Mechanical problems like breakage of the catheter balloon, the obstruction of a catheter, and the accidental yanking of the tube can cause while patient is using the foley catheters during specific medical conditions, so care must be taken. After using foley catheter for long time People may face issues like urinary retention (not able to completely empty the bladder) upon its removal.
Foley Catheters Classification of the Product:
Foley Catheters Regulatory Process Overview, By Country:
Food and Drug administration (FDA) has Centre for devices and radiological health (CDRH) division which is involved in the regulation of all the medical devices used in healthcare system for ensuring its safety, efficacy and quality.
FDA has issued a guidance document "Guidance for the Content of Premarket Notifications for Conventional and Antimicrobial Foley Catheters", providing complete guidance on the regulatory requirements in the approval process foley catheters in United States. FDA has classified Foley Catheters as Class II devices under 21 Code of Federal Regulation (CFR) 876.5130, and classification is specifically under product codes 78 EZL (balloon retention type) and 78 MJC (antimicrobial urological catheter and accessories).
Key Regulatory Requirements for Foley Catheters by U.S. Food and Drug Administration
- Premarket Notification (510(k)) Submission
Foley catheters are class II medical devices, so they require premarket notification 510 (k), which demand the substantial equivalence to a legally marketed predicate device, and the premarket notification must include information like:
- Device Name: Both trade and classification names.
- Establishment Registration Number: If applicable.
- Device Class and Panel: Class II, Gastroenterology/Urology panel.
- 510(k) Summary or Statement: Safety and effectiveness information.
- Labeling: Proposed labels, including intended use, directions for use, and any warnings or contraindications.
- Summary of Equivalence: Comparison with predicate devices.
- Device Description: Detailed physical description, including materials and dimensions.
- Performance Data: Functional and performance testing data.
- Sterility Information: Details on sterilization methods and packaging.
- Labeling Requirements
Labels used for the product must include following information on it:
- Intended Use Statement: Specific indications for use.
- Directions for Use: it should include Instructions on preparation of foley catheters, insertion, removal, and maximum indwelling time and procedures to use if the balloon fails to deflate, mention of single use or it is reusable.
- Warnings and Contraindications: Any relevant safety information.
- Antimicrobial Information: If applicable, details on the antimicrobial agent, including concentration, spectrum of activity, and potential for hypersensitivity reactions.
- Performance and Biocompatibility Testing
Manufacturers should provide accurate data related to performance and biocompatibility of the product gathered during preclinical and clinical testing, which is most important to maintain and ensure the safety or the device to the patient using it, which includes:
- Data related to performance characteristics like: Flow rate, Balloon integrity, and deflation characteristics.
- Cytotoxicity, irritation, and sensitization test for ensuring biocompatibility of the product material used.
- Antimicrobial Efficacy test to check Effectiveness in reducing urinary tract infections.
- Sterility and Shelf Life
Complete Information on sterility of the product should be included like: Sterilization Method utilised for the product, Validation of the sterilization process. Then packaging material and description of packaging and design to maintain and ensure sterility. Shelf-Life data, expiration date testing to check the effect of storage and adverse shipping conditions.
- Device Modifications
Any significant changes to the device from the predicate device must be supported by appropriate data to ensure that the modification does not affect the safety or effectiveness of the device.
FDA review of the premarket submission and decision on product approval
FDA has expert Committee who do thorough inspection of all the data submitted by the manufacturer seeking approval for its product including the premarket notification data, and if the device is meeting all the regulatory requirements and is up to standards set by the FDA, device is approved and allowed to be marketed.
FDAs Post market surveillance program
FDA ensure the safety and effectiveness of the device even after its approval and launch in the market to maintain its safety and effectiveness throughout its use by the patients, if any adverse events are reported during this post market surveillance, they immediately go for product recall action.
For instance, in December 2019, The U.S. Food and Drug Administration (FDA) has classified the recall of Boston Scientific's POLARx Cryoablation Balloon Catheters as the most serious type. The catheters are used in ablation, minimally invasive procedures to treat recurrent symptomatic atrial fibrillation. Reason for recall was due to a higher number of reports of esophageal injuries, mainly atrio-esophageal fistula, which was leading to severe complications like air bubbles blocking blood vessels in the brain. The FDA reported that the use of these catheters can cause stomach and intestinal bleeding, systemic infections, and even death. There were seven injuries reported, and four deaths related to these devices.
Foley Catheters Regulatory Updates:
Jun 2024, Silq Technologies has received an Innovative Technology contract from Vizient, Inc. for its ClearTract® Foley Catheter. This contract, awarded based on recommendations from hospital experts on Vizient’s customer-led councils, highlights the catheter's unique qualities that potentially improve healthcare. The ClearTract® Foley Catheter, which uses zwitterion technology to minimize biofilm formation, urinary tract infections, encrustation, and blockage, is FDA cleared for urethral, suprapubic, and nephrostomy applications. This technology aims to reduce additional healthcare costs associated with catheter-related complications and supports global antimicrobial management by reducing the need for antibiotics.
December 2023, BD (Becton Dickinson & Company) has signed an interim agreement to become the exclusive global license partner, excluding China, for Bactiguard-coated Foley catheters. This agreement focusses on its licensing business, important transformation is phase out of Bactiguard's medical device portfolio. The partnership, which extends BD's sales exclusivity to regions including the US, Japan, UK, Canada, Ireland, and Australia, aims to ensure that there are no supply disruptions faced by current distributors and customers.
Regulatory hurdles and Possible Risk in development of Foley Catheters:
Foley Catheters fall in the category of medical devices, and it needs premarket notification 510(k) submission, which requires evidence of substantial equivalence to the legally marketed product, failing to which will result in complications in acceptance of the product.
Complying with strict biocompatibility testing, material used in the catheter’s development should be proved to be safe in irritation, sensitization, cytotoxicity testing, proving to be safe and effective to be used by the patient.
Meeting international standards, failure to show compliance with evolving ISO standards, can block access to international markets and delay the regulatory approval process.
Failure to meet regulatory requirements can lead to voluntary product recalls, which significantly restrain the growth of the global market for dialysis catheters, urology catheters, and intravascular catheters. These recalls, often due to safety concerns like defects or potential complications, disrupt product availability and undermine consumer confidence. When manufacturers pull devices from the market, it can result in temporary shortages, increased costs for replacements, and delays in patient treatments.
Foley Catheters Competitive Landscape Dashboard:
Companies With Marketed Catheters Products
- Braun Melsungen AG (Germany)
- Cardinal Health (US)
- Coloplast Ltd. (Denmark)
- Boston Scientific Corporation (US)
- ConvaTec Inc. (UK)
Regulatory Landscape - Table of Content
Table of contents will appear here once available.
Customer Stories

“This is really good guys. Excellent work on a tight deadline. I will continue to use you going forward and recommend you to others. Nice job”

“Thanks. It’s been a pleasure working with you, please use me as reference with any other Intel employees.”

“Thanks for sending the report it gives us a good global view of the Betaïne market.”

“Thank you, this will be very helpful for OQS.”

“We found the report very insightful! we found your research firm very helpful. I'm sending this email to secure our future business.”

“I am very pleased with how market segments have been defined in a relevant way for my purposes (such as "Portable Freezers & refrigerators" and "last-mile"). In general the report is well structured. Thanks very much for your efforts.”

“I have been reading the first document or the study, ,the Global HVAC and FP market report 2021 till 2026. Must say, good info! I have not gone in depth at all parts, but got a good indication of the data inside!”

“We got the report in time, we really thank you for your support in this process. I also thank to all of your team as they did a great job.”