
Cardiovascular Devices Regulatory Landscape
Regulatory Landscape - Overview
Cardiovascular Devices Regulatory Landscape: Product Overview
Cardiovascular diseases comprise a group of diseases which affect heart and blood vessels. They include heart or blood vessels issues like narrowing blood vessels in heart, or in any other organ of the body, heart valves not working properly, or irregular heart rhythms.
As per WHO report cardiovascular diseases are considered as one of the major leading causes of death worldwide. There have been lots of advancements in the treatment options of cardiovascular diseases, and cardiovascular devices are the major advancement, enhancing the life of many patients with this disease.
These devices are very complex and used for the treatment of diseases with high risk, which calls for the need for strict regulatory framework, FDA plays a major role for ensuring the safety, efficacy and quality of the cardiovascular devices.
Cardiovascular Devices Types
Based on type of device used and purpose cardiovascular devices are segmented into Diagnostic & Monitoring, Therapeutic & Surgical Devices. Cardiovascular devices are of many types including catheters, stents, artificial heart valves, hemodialyzers, ventricular assist devices (VADs), endovascular grafts, and inferior vena cava (IVC) filters, and even devices that monitor and affect electrical activity including cardiac mapping systems, ablation catheters, pacemakers and defibrillators.
Cardiovascular Devices Applications
Catheters are used for diagnosis and treatment of many heart conditions, like irregular heartbeats- arrythmias, chest pain- angina, heart valve issues many more. Cardiovascular disorders are increasingly being treated using catheter-based procedures such as transcatheter mitral valve repair, transcatheter aortic valve replacement (TAVR), and others. These minimally invasive methods provide better patient outcomes, shorter hospital stays, and faster recovery periods. The inclination towards transcatheter treatments signifies a dedication to improving cardiovascular care while reducing procedural invasiveness.
Pacemakers are used to regulate the heartbeats rate, as a healthy heart has own pacemaker regulating heartbeats, in case of any heart disease if pacemaker don’t do regulation, artificial pacemakers can correct the issue, it is a small device battery operated, sending electrical impulses to the heart, maintaining a proper rate of heartbeat and rhythm.
Artificial Heart valves are used for replacing the diseased heart valve, to restore its function and overcome cardiovascular issues. A ventricular assist device (VAD) pumps blood from the lower chambers of the heart to the rest of the body, used for the treatment for a weak heart or failure of heart. usually used to continue heart normal function, until treatments, such as a heart transplant are available.
Hemodialyzers are used in medical procedures for artificially cleaning blood, involves passing of blood through a dialyzer, which is an artificial kidney, removing waste and excess water. Machine controls all flux of fluids involved in the treatment.
Endovascular grafts, also known as stent grafts, are medical devices used to reinforce weak and damaged blood vessels, treating aneurysms and dissections of major arteries, also are minimally invasive alternatives to open surgery, help to maintain blood flow and prevent rupturing of blood vessels.
The increasing popularity of wearable cardiac devices is one such development. Wearable technology, such as portable electrocardiogram (ECG) machines and heart rate-monitoring smartwatches, is enabling people to take charge of their cardiovascular health. This pattern allows for ongoing monitoring and early identification of cardiovascular problems, which is consistent with the larger movement in healthcare towards preventive care.
For instance, increasing product approval in the market space is boosting the market growth.
- March 2025, Powerful Medical's PMcardio STEMI AI ECG model has received the FDA Breakthrough Device Designation. This AI-driven technology helps detect ST-elevation myocardial infarction (STEMI) and its equivalents, which are serious heart conditions needing immediate treatment. The designation means the FDA will expedite the review process, recognizing the model's potential to improve heart attack diagnosis and treatment.
- October 2022 The HELIOSTAR Balloon Ablation Catheter was introduced in Europe by Biosense Webster, Inc., a subsidiary of Johnson & Johnson MedTech. When used with a compatible multi-channel RF generator, the HELIOSTAR Balloon Ablation Catheter is indicated for cardiac ablation as well as catheter-based cardiac electrophysiological mapping (stimulating and recording) of the atria.
Cardiovascular Devices Product Development Steps:
FDA has a center for devices and Radiological Health (CDRH) which is responsible for regulation of manufacturing, repackage, relabel, import of medical devices.

Fig- Cardiovascular device development and approval process.
Source: MRFR analyses
Cardiovascular devices Market trends
February 2025, Cardio Diagnostics has partnered with seven new provider organizations to expand the reach of its AI-driven precision cardiovascular medicine tests. These tests, Epi+Gen CHD and PrecisionCHD, are designed to improve the prevention, detection, and management of coronary heart disease. The new partnerships span various regions and medical specialties, highlighting the growing adoption of these advanced blood tests.
February 2024, X-trodes has received FDA 510(k) clearance for its Smart Skin solution, known as the X-trodes System M. This wireless wearable technology uses customizable dry-printed multimodal electrode patches to monitor various biopotential signals, including EEG (brain activity), EKG/ECG (cardiac monitoring), EOG (eye movement), and EMG (muscle activity). The FDA clearance follows an extensive scientific assessment that confirmed the system's accuracy and consistency, comparable to existing FDA-cleared clinical devices. This approval will help X-trodes pursue further validations for cardiovascular and sleep monitoring applications.
Cardiovascular Devices Market Size Overview:
As per MRFR analysis, the Cardiovascular Devices Market Size was estimated at 56.93 (USD Billion) in 2024. The Cardiovascular Devices Market Industry is expected to grow from 60.68 (USD Billion) in 2025 to 107.89 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 6.60% during the forecast period (2025 - 2034).Global elderly population growth, sedentary lifestyles, and the increasing frequency of chronic cardiac conditions, are the key market drivers enhancing the market growth.
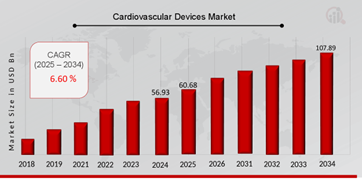
Source: The Secondary Research, Primary Research, MRFR Database and Analyst Review
Cardiovascular Devices Regulatory Landscape:
There are several key regulatory agencies who oversee the approval and monitoring of Cardiovascular Devices to ensure their safety, efficacy, and quality.
| Regulatory agencies | Regulatory Ministry |
| Federal Food and Drug Administration | United States: Department of Health and Human Services (HHS) |
| The Medicines and Healthcare products Regulatory Agency | United Kingdom: The Medicines and Healthcare products Regulatory Agency (MHRA) under the Department of Health and Social Care (DHSC) |
| Central Drug Standard Control Organization | India: The Ministry of Health and Family Welfare |
| South African Health Products Regulatory Authority (SAHPRA) | National Department of Health. |
| Pharmaceuticals and Medical Devices Agency (PMDA) | Japan: Ministry of Health, Labour and Welfare. |
| National Medical Products Administration (NMPA) | China: The Ministry of Health |
| Health Sciences Authority | Singapore: The Ministry of Health |
| European Medicine Agency | European union |
| Brazilian Health Regulatory Agency (Anvisa) | Ministry of Health, part of the Brazilian National Health System (SUS) |
Cardiovascular Devices Guidelines:
Eligibility: Cardiovascular devices like implantable devices, pacemakers, stents, artificial heart valves, endovascular grafts, catheters and many more are used for treatment of various heart related problems, for instance they are used in Patients with high risk of sudden cardiac arrest due to ventricular arrhythmias, patients with bradycardia (slow heart rate), individuals with heart block or patients with coronary artery diseases, or patients in need of heart transplant and many more heart conditions, enhancing their life expectancy. Older and high-risk patients are treated with minimally invasive devices.
Cardiovascular Devices Classification of the Product:
Cardiovascular Devices Regulatory Process Overview, By Country:
US FDAs Centre for Devices and Radiological Health (CDRH) is responsible for ensuring safety, efficacy and quality of the cardiovascular devices. It comprises several offices involved in different activities for medical device regulation. Office of product evaluation and quality (OPEQ) manages device evaluation, compliance and surveillance. The Office of Health Technology has an Office of cardiovascular devices, including subdivisions DHT2A, DHT2B, DHT2C under the office of product evaluation and quality (OPEQ) which oversees the regulatory requirements of cardiovascular devices, checking its compliance activities and approval and post market safety.
Office of Device Evaluation is involved in pre-market application reviews, pre-market approvals (PMAs) and exemptions for investigational device exemptions; then Office of Compliance is responsible for ensuring safety and efficacy through reviewing inspection reports for all medical devices on the market; the Office of Surveillance and Biometrics oversees adverse events (AE) reports for medical devices and related post-marketing activities.
Cardiovascular devices fall under the classification of medical devices given by FDA, 21 code of federal regulation (CFR) part-870 has regulations for cardiovascular devices.
US FDA approved various medical devices for cardiovascular treatment, like automated external defibrillators (AEDs), cardiac ablation catheters, cardiovascular angioplasty devices, cardiac pacemakers, implantable cardioverter defibrillators (ICDs), prosthetic heart valves, stents, and ventricular assist devices (VADs), they FDA classify these medical devices into 3 classes:
- Class I: include devices of lowest risk to patients, needs only General Controls for regulatory approval enough to ensure its safety and efficacy, where manufacturers do device registration with the FDA and maintain compliance with relevant guidelines.
- Class II: include devices causing moderate risk to patients and need the general and special controls are to confirm its safety and efficacy for getting regulatory approval, regulatory clearance is obtained through the Premarket Notification 510(k) pathway. 80% of devices that receive 510(k) clearance are Class II medical devices.
- Class III: include devices of the highest risk, they usually include life sustaining, supporting, and long-term implantable devices in their category, they need pre-market approval (PMA) for regulatory approval. cardiac pacemakers and ablation catheters are examples of Class III devices. As Class III devices have a strict regulatory framework as they pose the highest risk to patients.
FDA offers two alternatives’ options to traditional submission 510(k): which include special 510(k) and abbreviated 510(k) pathways, to speed up the approval process for medical devices.
- Special 510(k)- for devices which are modifications of a manufacturer’s own legally marketed device
- Abbreviated 510(k)- streamlining the review process by using FDA guidance documents, special controls, or consensus standards to demonstrate substantial equivalence.
For identifying and tracking medical devices throughout their lifecycle, Unique Device Identification (UDI) system, established by the FDA Amendments Act of 2007 and further defined by the Food and Drug Administration Safety and Innovation Act of 2012, plays a crucial role.
Examples of few cardiovascular devices falling in different medical classes;
| Cardiovascular device | Class |
| Temporary pacemaker electrode | Class II |
| Echocardiograph | Class III |
| Implantable pacemaker pulse generator | Class III |
| Carotid sinus nerve stimulator | Class III |
List of recently approved Cardiovascular Devices;
| Cardiovascular device | Company | Category | Date of Approval |
| MYNX CONTROL Venous Vascular Closure Device (VCD) | Cordis US Corporation | Vascular Closure Delivery System | June 2024 |
| Edwards EVOQUE Tricuspid Valve Replacement System | Edwards Lifesciences LLC | Tricuspid Valve Replacement System | February 2024 |
| TZ Medical Adult and Pediatric Multi-Function Defibrillation Electrodes and Adaptors | TZ Medical, Inc | Defibrillator | January 2024 |
| Edwards SAPIEN 3, SAPIEN 3 Ultra, and SAPIEN 3 Ultra RESILIA Transcatheter Heart Valve System | Edwards Lifesciences LLC | Heart Valve | May 2024 |
Cardiovascular Devices Regulatory Updates:
January 2025, according to the American College of Cardiology, new guidelines for cardiac implantable electronic devices (CIEDs) have been published. These guidelines cover devices like ICDs, CRT, and pacing, addressing various clinical scenarios and new technologies. These sections cover a range of clinical scenarios, from primary and secondary prevention ICDs to new technologies like leadless pacing and conduction system pacing. The guidelines also address heart failure scenarios, including left ventricular assist devices (LVADs) and devices following cardiac transplantation. The document emphasizes the importance of shared decision-making between clinicians and patients to incorporate individual patient values and preferences. It also highlights that clinical judgment is necessary for assessing device implantation for individual patients.
March 2025, according to the European Society of Cardiology, they have called for a revision of the Medical Device Regulation (MDR) due to significant challenges in its implementation. The MDR was intended to improve the safety and quality of medical devices in the EU, but it has led to increased regulatory complexity and high certification costs, reducing the availability of essential devices. The ESC highlights the need for more comprehensive provisions, including better involvement of clinical experts and a centralized governance system. They also recommend special regulatory pathways for breakthrough, orphans, and pediatric devices.
Cardiovascular Devices Regulatory Challenges:
Complex Approval Processes: cardiovascular devices need to go through a strict regulatory framework to get the approval like strict classification and documentation requirements, which is quite challenging.
High Costs: To ensure compliance involves high cost is required for clinical trials, quality assurance, and manufacturing upgrades.
Limited Infrastructure: lacks sufficient testing facilities and regulatory resources further delay the approval process.
Dynamic Regulations: Frequent updates to medical device guidelines require constant adaptation, posing challenges for manufacturers to stay compliant.
Possible Risk in the development of Cardiovascular Devices:
Financial cost - Research and development process of developing cardiovascular devices is highly expensive, and low sales of launched products in market will further cause problems in manufacturing. For instance, Abbott has discontinued global sales of Absorb bioresorbable vascular scaffold in 2017 due to low commercial sales, highlighting financial risk associated with product performance and market acceptance.
Product recall - products may fail in the regulatory process or in post-market surveillance due to adverse events reporting. For instance, FDA issued notice of recall, with 444 complaints about the device, the most serious type of recall, of Medtronic’s certain implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy (CRT-D) due to unexpected rapid battery depletion, caused by short circuit emphasizing technological and manufacturing challenges.
Cardiovascular Devices Competitive Landscape Dashboard:
Companies With Marketed Cardiovascular Devices Products:
- B Braun Melsungen AG
- Abbott
- Johnson & Johnson Services Inc.
- Boston Scientific Corporation
- LivaNova Plc
- Edwards Lifesciences Corporation
- GE Healthcare
- Medtronic
- Siemens Healthcare GmbH
- Terumo Cardiovascular Systems Corporation
Regulatory Landscape - Table of Content
Table of contents will appear here once available.
Customer Stories

“This is really good guys. Excellent work on a tight deadline. I will continue to use you going forward and recommend you to others. Nice job”

“Thanks. It’s been a pleasure working with you, please use me as reference with any other Intel employees.”

“Thanks for sending the report it gives us a good global view of the Betaïne market.”

“Thank you, this will be very helpful for OQS.”

“We found the report very insightful! we found your research firm very helpful. I'm sending this email to secure our future business.”

“I am very pleased with how market segments have been defined in a relevant way for my purposes (such as "Portable Freezers & refrigerators" and "last-mile"). In general the report is well structured. Thanks very much for your efforts.”

“I have been reading the first document or the study, ,the Global HVAC and FP market report 2021 till 2026. Must say, good info! I have not gone in depth at all parts, but got a good indication of the data inside!”

“We got the report in time, we really thank you for your support in this process. I also thank to all of your team as they did a great job.”