
Regulatory Landscape - Overview
Oncology Drugs Regulatory Landscape: Product Overview
In the evolving healthcare sector, the development and approval of oncology drugs plays very important role. These are therapeutic preparations designed for the treatment of various types of cancers.
Adherence to the regulatory guidelines for the development of these drugs is most important for ensuring the safety and efficacy of the drugs. The office of oncologic diseases (OOD) as a part of FDA’s Centre for drug evaluation and research (CDER) supervises the development, approval and regulation of drugs for cancer and hematologic malignancies. OOD is responsible for making sure that these drugs and biologics to treat cancer are safe and effective for the U.S. public. In past 20 years about 150 drugs have been approved by US FDA, which include 42 novel therapeutics.
According to the American Association of Cancer Research, in 2024 FDA has issued more than 60 new oncology drug approvals, including 11 first-in-class therapeutics. Two new oncology medicines, Rajamanis (Revuforj) and Zolbetuximab, were approved by FDA for the treatment of leukemia and gastric/ gastroesophageal junction cancer which has introduced new targets for cancer treatments.
Oncology Drugs Type
There are different types of cancer treatments, patients receive either single treatment or combinations of treatments based on nature of the cancer. Different types of oncology therapeutics used for cancer treatments includes cytotoxic or chemotherapy drugs (alkylating agents, antimetabolites), Targeted drugs (monoclonal antibodies, cancer growth blockers), Hormonal drugs, immunotherapy drugs.
Targeted therapy
- It is a type of cancer treatment which focuses on the specific proteins or molecules which are involved in the cancerous cell’s growth, division and spread, making the treatment more precise and often reducing the side effects.
- These therapies can be administered alone or in combination with other treatments such as radiation therapy, chemotherapy or surgery. Mode of administration can be orally as a pill or capsule or intravenously (directly into the vein).
Form/ ingredients used
Targeted therapies either use small molecule drugs or monoclonal bodies.
Monoclonal antibodies are lab made molecules or therapeutic molecules, which are designed to bind to specific targets found on cancer cells. Examples include Bevacizumab, Trastuzumab developed by Genentech. They mark the cancer cells so they can be seen and destroyed by the immune system. Add more examples
Small molecule drugs are so small that they can enter the cancerous cells and block the proteins involved in cancerous cells growth. Examples of small molecule drugs include tyrosine kinase inhibitors such as imatinib, developed by Novartis and Osimertinib developed by AstraZeneca.
Oncology Drugs Mode of action
Targeted therapies treat cancer by disrupting specific proteins which allows tumors to grow and spread throughout the body. some of the substances that become “targets” in Targeted therapy for cancer includes overexpressed proteins in cancerous cells, tumor specific antigens, mutated proteins, Genomic alterations.
Targeted drugs work by blocking or turning off chemical signals that instruct cancerous cells to grow, changing the proteins in cancerous cells so the cells die, stopping formation of new blood vessels required for feeding cancer cells, triggering the immune system to kill the cancer cells, carry chemicals or radiations along with them to target site to kill the cancerous cells.
Examples of these therapies include:
- Angiogenesis inhibitors: they block the formation of new blood vessels required for feeding and nourishing cancerous cells, for example, bevacizumab (approved for treatment of different cancers such as Cervical Cancer, Colorectal Cancer, Glioblastoma).
- Proteasome inhibitors: They inhibit specific enzymes required for breaking down of proteins in cells, so the cancer cells are instructed to die. Example includes, bortezomib (approved for treatment of multiple myeloma).
- Signal transduction inhibitors: They can disrupt cellular signals, so which change the actions of the cancer cell. Example includes, imatinib (approved for treatment of certain chronic leukemias).
Oncology Drugs Applications
Targeted therapies used for cancer treatment are specifically designed to target genetic mutations or overexpression of proteins found in tumor cells or normal genes of individual patient, so it is kind of personal treatment plan given to patient based on type of cancer patient is having. Thus, the use Targeted therapies is often referred to as a “precision medicine” or “precision oncology.”
For lung cancer targeted therapies showed as a most effective treatment option. In lung cancer selection of once treatment to work for all patients is not possible since abnormal cancer cells are different in every patient, here Targeted therapies work efficiently aiming at those differences.
Some of the targeted therapy drugs approved by FDA for treatment of cancers includes:
- Targeted therapy drugs approved for cervical cancer
- bevacizumab (Avastin)
- pembrolizumab (Keytruda)
- tisotumab vedotin-tftv (Tivdak)
- Targeted therapy drugs approved for colorectal cancer
- adagrasib (Krazati)
- bevacizumab (Avastin)
- cetuximab (Erbitux)
- Targeted therapy approved for breast cancer
- abemaciclib (Verzenio)
- ado-trastuzumab emtansine (Kadcyla)
- alpelisib (Piqray)
Alternative to targeted therapy can be immunotherapy, targeted therapy works by directly attacking on cancerous cells, and on the other side immunotherapy works by activating immune system to identify and fight cancer, it is effective for melanoma, triple negative breast cancers, providing long term survival benefits.
Also, radiation therapy is widely used to treat various cancers, and it works by destroying the DNA of cancer cells by using ionizing radiation to kill the cancerous cells preventing the growth and spread of cancer in patient’s body. It is also used in combination with other therapies such as immunotherapy. And the gamma rays or x-rays are used as ionizing rays to destroy cancer cells.
Oncology Drugs Product Development Steps:
FDA Approval Process for Oncology Drugs
The FDA has formed four methods to speed up the development of new drugs: Fast Track, Breakthrough Therapy, Accelerated Approval (AA), and Priority Review.
List Of Breakthrough Therapy Designated Oncology Drugs for Targeted and Immunotherapy
| Drug | Company | Approval | Details |
| inavolisib (GDC-0077) + palbociclib (Ibrance) and fulvestrant | Roche | 5/21/2024 | Targeted therapy for the Treatment of PIK3CA-mutated breast cancer. |
| NVL-655 | Nuvalent | 5/20/2024 | Targeted therapy for the Treatment of patients with locally advanced or metastatic ALK-positive non-small cell lung cancer (NSCLC) who have been previously treated with two or more ALK tyrosine kinase inhibitors (TKIs). |
| Anktiva | Immunity Bio | 4/22/2024 | Immunotherapy for the Treatment of BCG-Unresponsive Non-Muscle Invasive Bladder Cancer |
| Cretostimogene Grenadenorepvec | CG Oncology | 12/5/2023 | Immunotherapy for the high-risk Bacillus Calmette-Guérin (BCG)-unresponsive Non-Muscle Invasive Bladder Cancer (NMIBC) with carcinoma in situ with or without Ta or T1 (papillary) tumors. |
Source: Friends of Cancer Research
Preclinical Research and Development:
- Discovery and target identification
- Animal Testing and Toxicological Study
Investigational New Drug Application
Clinical Trials:
- Phase 1-safety and dosage
- Phase 2-Efficacy And Side Effects
- Phase 3-Comparative Studies
New Drug Application and Biologics license applications
Advisory Committee Meetings
US FDA Decision
Post Market Surveillance
Oncology Drugs Market Size Overview:
As per MRFR analysis, the Oncology Drugs Market Size was estimated at 223.69 (USD Billion) in 2024. The Oncology Drugs Market Industry is expected to grow from 248.62 (USD Billion) in 2025 to 541.71 (USD Billion) till 2035, at a CAGR (growth rate) is expected to be around 8.10% during the forecast period (2025 - 2035). Increased cancer incidence and prevalence and advancements in cancer research and drug development are the key market drivers enhancing the growth of the market.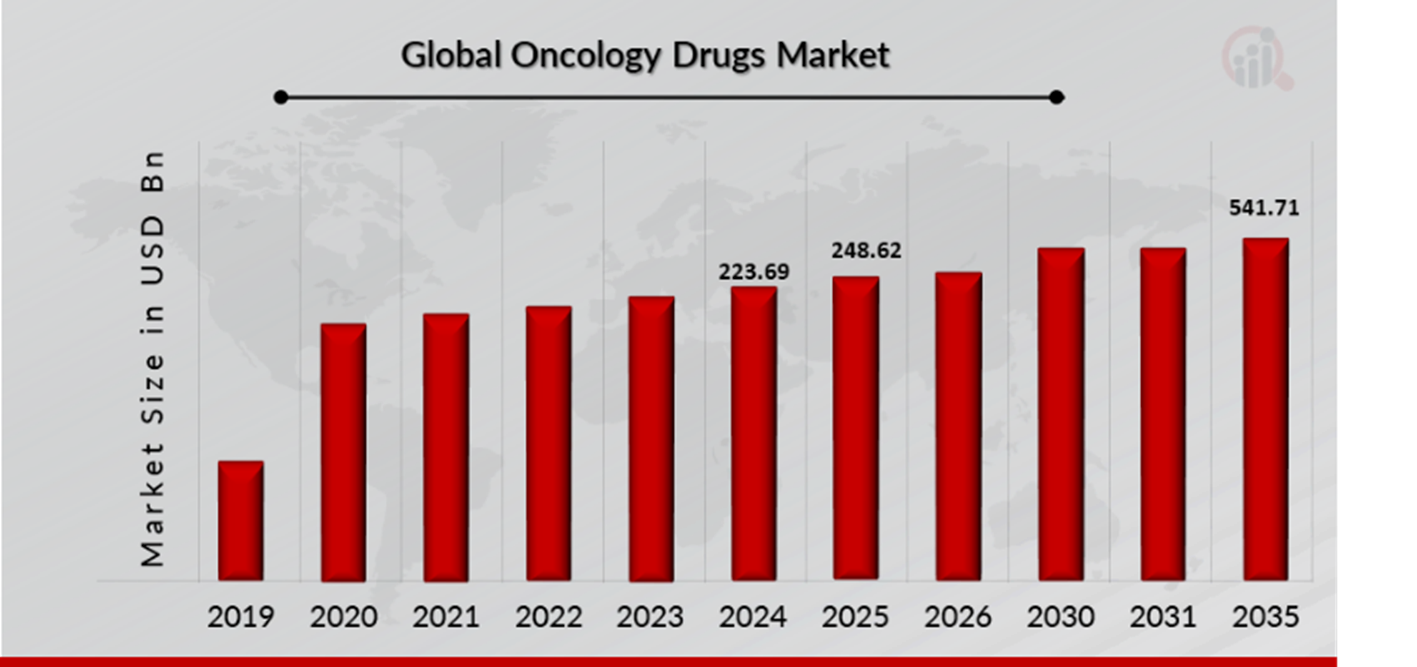
Source: Secondary Research, Primary Research, MRFR Database and Analyst Review
Oncology Drugs Regulatory Landscape:
There are several key regulatory agencies oversee the approval and monitoring of oncology drugs to ensure their safety, efficacy, and quality.
| Regulatory agencies | Regulatory Ministry |
| Federal Food and Drug Administration | United States: Department of Health and Human Services (HHS) |
| The Medicines and Healthcare products Regulatory Agency | United Kingdom: The Medicines and Healthcare products Regulatory Agency (MHRA) under the Department of Health and Social Care (DHSC) |
| Central Drug Standard Control Organization | India: The Ministry of Health and Family Welfare |
| Health Canada | Canada: The Ministry of Health |
| Pharmaceuticals and Medical Devices Agency (PMDA) | Japan: Ministry of Health, Labour and Welfare. |
| National Medical Products Administration (NMPA) | China: The Ministry of Health |
| Health Sciences Authority | Singapore: The Ministry of Health |
| European Medicine Agency | European union |
| Therapeutic Goods Administration (TGA) | Commonwealth of Australia |
Oncology Drugs Guidelines:
Eligibility:
In oncology treatment, personalized medicine or precision oncology depends on the genomic sequencing of the patient’s tumor, precision medicine usually focuses on a specific genetic aberration within cancer as a target, companion diagnostics is a test for biomarker testing done to choose the best suitable treatment for the patient which will show least possible side effects and great effect of the drug on them.
Targeted therapy needs to match to the genes of the patient or particular cancer type, for some cancers patients have the target for the targeted therapy, which is found out using the companion diagnostic test, targeted therapy is also administered to the patient in case they are not responding to other treatments or the cancer has spread.
Oncology Drug Types:
Targeted therapy uses Targeted drugs such as monoclonal antibodies, or small molecule drugs for the treatment of cancer.
Dosage:
Most targeted therapy is administered to the patients via oral drugs such as capsules or pills, or administered intravenously, that is directly into the vein. And like other cancer treatments targeted therapy is also given in cycles, that is period of treatment followed by period of rest form.
- Imatinib and dabrafenib are oral targeted therapy drug,
- Bevacizumab, Trastuzumab are given intravenously.
Oncology Drugs Classification of the Product:
Oncology Drug Regulatory Process Overview, By Country:
FDA Approval Process for Oncology Drugs
FDA approval indicates that drug has gone through strict testing to ensure its safety and efficacy, this approval promotes trust among healthcare professionals and patients.
The FDA has formed four methods to speed up the development of new drugs and regulatory submissions: Fast Track, Breakthrough Therapy, Accelerated Approval (AA), and Priority Review.
Preclinical Research and Development:
Discovery and target identification: Initial step is to find molecular targets responsible for cancer growth, this is a foundation step for drug development.
Animal Testing and Toxicological Study: Before conducting clinical trials on humans, extensive animal testing is done to evaluate the safety and efficacy of the developed drug.
99% of human genes are similar to the mice genome, therefore 95% in vivo cancer studies use mice for the preclinical studies of oncology drugs.
There are four most widely used methods for constructing mouse cancer models:
- chemically induced model
- cell line-derived xenograft (CDX) model
- patient-derived xenograft (PDX) model
- genetically engineered mouse model (GEMM).
Many drugs with good therapeutic effect in preclinical studies, don’t play corresponding role in the tumor patients, this is due the differences in the immune systems among the mammals which don’t allow the existing models to accurately predict the interaction between the human immune system and the tumor
Therefore, there is a development of humanized mouse models, and it is divided into 3 categories:
- Hu-BLT (human bone marrow, liver and thymus) model
- Hu-HSCs (human hematopoietic stem cell) model
- Hu-PBL (human peripheral blood lymphocyte) model
Investigational New Drug Application:
If preclinical studies show promising results, pharmaceutical company submits an investigational new drug (IND) application to FDA, detailing drugs potential and safety data.
Clinical Trials:
Phase 1-safety and dosage: This is a small-scale trial on healthy volunteers to check the safety and dosage levels of the drug, this trial in undertaken for several months.
Phase 2-Efficacy And Side Effects: It is a large-scale trial undertaken on real patient population to evaluate the Efficacy and side effects of drugs.
Phase 3-Comparative Studies: Undertaking trials on larger patient population, comparative step of the developed drug and existing treatments, to check its superiority.
New Drug Application and Biologics license applications:
Upon successful completion of clinical trials, New Drug Application (NDA) pertain to traditional small molecule drugs, is submitted to FDA, providing comprehensive data on drugs safety and efficacy. This submission is the key step in getting the drug approved.
BLA is a request to approve the biological product for interstate commerce. It contains all the information of the development process and demonstrate the biological products safety, purity and potency, as well as it contains proposed packaging and labelling information of the drug. As per BPCI act of 2009, all biologics must be approved under BLA pathway and licensed under Section 351 of the Public Health Service (PHS) Act, in addition to being regulated by the FD&C Act.
Advisory Committee Meetings:
An Independent panel of experts meets to recommend drug approval. Their expertise and advise help the FDA make decisions.
FDA Decision:
FDA Makes Decisions Based on Drugs Risks and Benefits, If Approved, Drug Moves to The Next Step of Manufacturing, Labeling, And Distribution Preparations.
Post Market Surveillance:
FDA monitors the drug even after its approval for ensuring drugs safety and efficacy
Oncology Drugs Regulatory Updates and Amendment’s:
November 20, 2024: Stakeholders want changes to FDA’s oncology multiregional trial guidelines, they want FDA to consider the logistical or legal challenges researchers face while conducting trials across different regulatory jurisdictions in its guidance on multiregional clinical trials (MRCTs) for oncology drugs. Also, they demanded clarification in regulatory perspective on the statistical methods which researchers may use, including Bayesian methods.
- It is difficult to get diversity in small patient group, so they demanded clear guidelines on statistical or qualitative thresholds.
- They also mentioned about logistical challenges and early phase trials and suggested FDA to provide scenarios or cancer types, where limited geographic representation will be accepted, particularly for initial data generation that may focus on proof-of-concept.
- ACRO requested clear guidelines for submitting premarket applications to multiple regulatory agencies at the same time.
February 16, 2024: FDA has granted accelerated approval for First Tumor-infiltrating Lymphocyte Therapy for the treatment of melanoma, it is a new type of immunotherapy and named as lifileucel.
Oncology Drugs Regulatory Challenges:
Regulatory challenges encountered during clinical trials are as follows:
| Therapy/problem | Challenge | Possible solution |
| Dose identification | Availability of animal models for targeted therapeutics such as monoclonal antibodies | Take minimum number of patients for small rapid human trial to identify the dose |
| Relevant patient population | Regular clinical trials often recruit patients with advanced/metastatic cancer and in these cases the immune response may be hindered in them | Considering heterogeneous population of patient, that is patients having with minimum disease burden and or advanced/metastatic disease |
| Personalized therapy | Each trial patient will have unique molecular targets, this makes it difficult for getting the sufficient sample size, required for approval | A flexible approach may allow limited approval for highly effective treatments in specific patient groups |
| Study design | All new products can’t be tested for superiority; non inferiority trials have their own challenges | Adaptation of study design from a case-to-case basis, and discussing it with the regulators for adopting relevant study design will help |
| Relevant endpoint selection | Regular cancer trials use overall survival OS as endpoint, requiring long follow-ups and a large sample size. | conditional approval by EMEA by adoption of other endpoints like PFS and ORR or accelerated approval by FDA |
| Choice of comparator/control or placebo group | Regulators require a comparator or control or placebo group which has proven efficacy or proven approval, but it is not always available for novel products. Also, definition of proven efficacy varies, and use of placebo in oncology trials is not always ethical. | Discussing with the regulators before the trial starts about the selection of the control/comparator or placebo group which will help to resolve this issue on a case-to-case basis. |
| Bias | For all the immunotherapeutic products, blinding may not always be possible | Discussing with regulators about the assessment for bias, mostly at the end of phase 2 trials, which will prevent hindrances in getting approval in future. |
Possible Risk in development of Oncology Drugs:
Withdrawal of Drugs: Due to the stringent regulatory rules there is a risk of drug not complying with the regulatory framework in clinical trials if the efficacy, quality, and safety of drug was not up to the standards which can results in withdrawal of the drugs from market.
Accelerated approval: This is beneficial for getting the treatments ready for patients faster, but it is challenging in recruiting the confirmatory trial and in meeting the clinical endpoints in drug regulation. The Food and Drug Omnibus Reform Act of 2022 needs the confirmatory trial to be ongoing at the time of approval and also it allows the Food and Drug Administration (FDA) to reject applications which are not having confirmatory trials.
Manufacturing risks: healthcare workers in their routine may face the risk during production of these drugs as they are carcinogenic. Lack of in-house facilities, regulatory compliance, distribution challenges, adapting to market demand. Use of suitable animal models and selection of suitable patients during the pre-clinical and clinical trials.
Competition: Since there is a rapid advancement in the oncology research also there are lots of regulatory complexities, there is always a risk of quick replacement of current treatment with new treatment with superior effect and least side effects. Competitors who get faster regulatory approvals or breakthrough or fast track designations have the benefits in the market compared to those who spend a lot of time in getting approvals for regulatory submissions.
Risk mitigation strategies for Oncology Drugs:
Preclinical studies: Extensive preclinical testing must be done for the early identification of the possible toxicities of the drug. Also, selection of animal model must be done very carefully, humanized animal models should be selected preferably which will mimic the human cancers closely.
Clinical trial designs: Selection of patients for the clinical trials must be done very carefully based on tumor type, genetic markers etc. And designs of the clinical trial must be flexible for dosage optimization process and optimal treatments.
Safety and quality monitoring: In between safety review must be taken frequently during the trials, adhering to good manufacturing practices to ensure the quality of the drug and development of strategies to identify, mitigate and manage potential safety concerns should be done.
Following regulatory framework: for ensuring the safety and efficacy for the drug, strictly doing oncology drug regulatory review and communicating regularly with regulatory agencies throughout the development process will help in successfully developing the drug.
Oncology Drugs Competitive Landscape Dashboard:
Companies With Marketed Oncology Drugs Products
- BRISTOL-MYERS SQUIBB COMPANY
- HOFFMANN-LA ROCHE LTD
- MERCK KGAA
- AMGEN INC.
- ELI LILLY AND COMPANY
- JOHNSON & JOHNSON SERVICES, INC
- ASTRAZENECA
- PFIZER INC
- NOVARTIS AG
- MERCK & CO., INC.
- SANOFI
Regulatory Landscape - Table of Content
Table of contents will appear here once available.
Customer Stories

“This is really good guys. Excellent work on a tight deadline. I will continue to use you going forward and recommend you to others. Nice job”

“Thanks. It’s been a pleasure working with you, please use me as reference with any other Intel employees.”

“Thanks for sending the report it gives us a good global view of the Betaïne market.”

“Thank you, this will be very helpful for OQS.”

“We found the report very insightful! we found your research firm very helpful. I'm sending this email to secure our future business.”

“I am very pleased with how market segments have been defined in a relevant way for my purposes (such as "Portable Freezers & refrigerators" and "last-mile"). In general the report is well structured. Thanks very much for your efforts.”

“I have been reading the first document or the study, ,the Global HVAC and FP market report 2021 till 2026. Must say, good info! I have not gone in depth at all parts, but got a good indication of the data inside!”

“We got the report in time, we really thank you for your support in this process. I also thank to all of your team as they did a great job.”