
Regulatory Landscape - Overview
Acne Drugs Regulatory Landscape: Product Overview
Acne a skin condition affecting several patient popluation worldwide, mostly seen in adolescent age group and young adults. Growing incidence of acne is leading to the development of new drugs or therapeutics for its treatment. For development of new drugs adhering to regulatory guidelines for ensuring safety and efficacy is crucial.
Based on severity, Acne is categorized into 3 levels: mild, moderate, and severe acne. Based on the size and depth of penetration they are divided into different types including Blackheads (open comedones), Whiteheads (closed comedones), Pimples (inflamed closed comedones), Raised, solid bumps (papules), Surface bumps containing pus (pustules), Deeper, firm bumps containing pus (nodules), Larger pockets containing pus (cysts), Sometimes even larger, deeper pockets containing pus (abscesses).
As per Pharmaphorum report, about 50 million people are suffering with acne skin problem in the US, and they are mostly the adolescent age group. Acne is scientifically known as Acne vulgaris, is caused due to clogging hair follicle in the skin, usually this appears on face but may also appear on back, chest or shoulders. According to the Journal of American Academy of Dermatology (JAAD) 1 person in every five people is affected by acne worldwide.
Under office of immunology and inflammation (OII) of FDA there is Division of Dermatology and Dentistry (DDD) which is involved in regulating new drug applications (NDA), Investigational new drug applications (IND) and Biologics Licensing Applications (BLAs) of drugs and biological products developed for the treatment of dermatology and dental conditions, including regulation of acne therapeutics.
Acne treatment seeks to minimize lesions, prevent scarring, and enhance the overall appearance of the skin, thereby increasing self-confidence in people affected.
Acne Drugs Types:
By therapeutic type acne is divided into 3 types: Oral (Antibiotics, Contraceptives, Retinoids, Hormonal), Topical (Antibiotics, Retinoids, Benzoyl Peroxide, Salicylic Acid, Azelaic Acid, Others), Systemic (Antibiotics, Intralesional, Steroid Injections) and based on prescription type it is divided into Prescription Based and Over the Counter (Non-Prescription Based).
Oral Antibiotic – Treatment for Acne
They are commonly used therapeutics to prevent or treat acne, generally used for the treatment of mild or severe acne conditions.
Form/ ingredients used
Oral antibiotics used for the treatment of acne include macrolides (erythromycin, clindamycin, azithromycin and roxithromycin), fluoroquinolones (levofloxacin), tetracyclines (doxycycline, minocycline and lymecycline) and co-trimoxazole.
Acne Drugs Mode of action:
Doxycycline from Tetracycline class of oral antibiotics is the most commonly used antibiotics for acne treatment. Each of this tetracycline drugs have same mode of action.
These drugs are transported at the lipid environment of pilosebaceous follicle in dermis, area where p. acne colonizes. Drug binds to the 30S ribosomal unit and blocks protein synthesis. There is reduction of neutrophil chemotaxis and inhibition of proinflammatory cytokines and matrix metalloproteinases. These drugs also have anti-inflammatory properties along with antibacterial function.
Acne Drugs Applications:
Mild acne can be treated with over-the-counter topical therapies that contain benzoyl peroxide, salicylic acid, or retinoids, which reduce inflammation, clear clogged pores, and inhibit bacterial development.
In more severe situations, oral drugs such as antibiotics (e.g., doxycycline) or hormonal treatments (e.g., birth control pills) may be administered to regulate oil production or alleviate underlying inflammation.
Stronger therapy, such as oral isotretinoin, may be used for persistent or cystic acne. These treatments address the underlying causes of acne, such as hyperactive oil glands and bacterial development, by lowering oil production and increasing skin cell turnover.
Additionally, light-based therapies or chemical peels can be utilized to cure acne scars and enhance skin texture.
Acne Drugs Product Development Steps:
FDA Approval Process for Acne Drugs
FDA approval indicates that drug has gone through strict testing to ensure its safety and efficacy, this approval promotes trust among healthcare professionals and patients.
The FDA has formed four methods to speed up the development of new drugs and regulatory submissions: Fast Track, Breakthrough Therapy, Accelerated Approval (AA), and Priority Review.
Some of the drugs approved by FDA recently include
-
April 2024: Allergan Aesthetics, a part of AbbVie, has recently expanded its SkinMedica line with the introduction of two innovative products aimed at treating acne-prone skin. The SkinMedica Acne Clarifying Treatment and SkinMedica Pore Purifying Gel Cleanser feature a formulation that includes 2% encapsulated salicylic acid, a key ingredient known for its effectiveness in combating breakouts.
-
May 2024: Zydus Lifesciences Ltd received final approval from the US Food and Drug Administration (USFDA) to market its generic version of Dapsone gel (7.5%) for acne treatment. The product will be manufactured at the company's topical manufacturing facility in Changodar, Ahmedabad.
-
October 2023:US FDA Approves IDP-126 (CabtreoTM), First Triple-Combination Drug for Acne by Bausch Health, for treating acne patients aged 12 and above.
-
in July 2022, Glenmark Pharmaceuticals has launched MINYM gel, a novel acne treatment, in India. This is India's first topical Minocycline 4% Gel for acne treatment.
-
In February 2020, Sun Pharmaceutical Industries Ltd. launched ABSORICA LD (Isotretinoin) capsules which is available in the US to treat severe, resistant nodular acne in patients aged 12 and up.
Acne Drugs Market Size Overview:
As per MRFR analysis, the Acne Drugs Market Size was estimated at 9,654.86 (USD Million) in 2024. The Acne Drugs Market Industry is expected to grow from 9,654.86 (USD Million) in 2025 to 12,714.54 (USD Million) till 2035, at a CAGR (growth rate) is expected to be around 2.79% during the forecast period (2025 - 2035). Increased acne incidence and prevalence and advancements in acne research and drug development are the key market drivers enhancing the growth of the market.
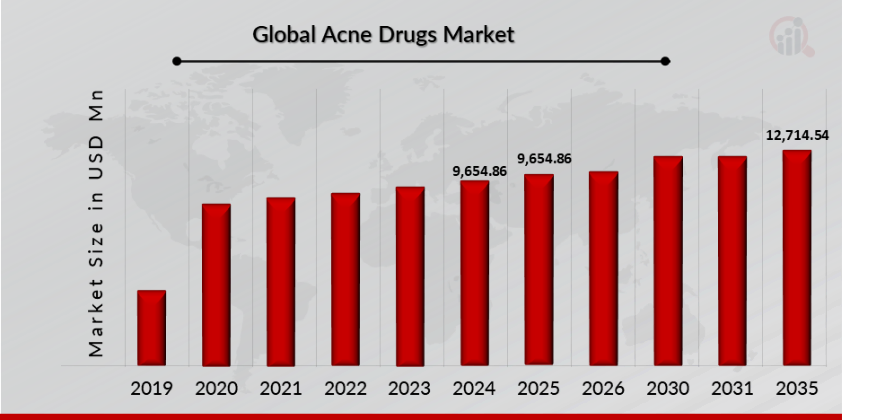
Source: The Secondary Research, Primary Research, MRFR Database and Analyst Review
Acne Drugs Regulatory Landscape:
There are several key regulatory agencies oversee the approval and monitoring of acne drugs to ensure their safety, efficacy, and quality.
| Regulatory agencies | Regulatory Ministry |
| Federal Food and Drug Administration | United States: Department of Health and Human Services (HHS) |
| The Medicines and Healthcare products Regulatory Agency | United Kingdom: The Medicines and Healthcare products Regulatory Agency (MHRA) under the Department of Health and Social Care (DHSC) |
| Central Drug Standard Control Organization | India: The Ministry of Health and Family Welfare |
| Health Canada | Canada: The Ministry of Health |
| Pharmaceuticals and Medical Devices Agency (PMDA) | Japan: Ministry of Health, Labour and Welfare. |
| National Medical Products Administration (NMPA) | China: The Ministry of Health |
| Health Sciences Authority | Singapore: The Ministry of Health |
| European Medicine Agency | European union |
| Therapeutic Goods Administration (TGA) | Commonwealth of Australia |
Acne Drugs Guidelines:
Eligibility: Acne is mostly found affecting the adolescent age group and young adults, but sometimes people at the age of 40 to 50 or above, also face this skin condition. Severe acne can leave permanent scars on the body, if not treated properly. Dermatologists suggest suitable treatment for the affected patient based on the type of acne the patient is having and its severity.
Acne Drug Types: Acne treatment drugs are divided into 3 types based on the type of therapeutic, which includes oral, topical or systemic.
Dosage: Repetitive use of these antibiotics at low doses for long time period during treatment process can lead to resistance of these drugs over time which results in limited use of these drugs. Therefore, treatment duration should not exceed more than 12 weeks.
European guidelines recommend lymecycline and doxycycline as systemic antibiotics and limit treatment period to 3 months to prevent development of resistance to drugs.
Classification of the Product:
Acne Drug Regulatory Process Overview, By Country:
Clinical Trial Design Features, as per guidance prepared by the Division of Dermatology and Dental Products in the Center for Drug Evaluation and Research at the Food and Drug Administration, includes:
-
Enrollment Criteria
According to this criterion the study population will be defined by the following things;
-
Minimum number of each lesion type: inflammatory and noninflammatory.
-
Baseline score, consistent with baseline lesion counts, on an Investigators Global Assessment (IGA).
-
Enrollment age, reflecting start of acne relative to adrenarche.
-
Study Design and Efficacy Endpoints
For assessment of effectiveness, following things should be considered by the sponsors;
-
Recommendation for conducting randomized, double-blind, properly controlled trials which include a placebo arm.
-
Acne can occur on face or trunk, but efficacy assessment should be restricted to face as it is the most frequent site of disease. For topical drug products, it can be applied to all affected areas during clinical trials.
-
Recommendation for IGA to be static evaluation of overall acne severity.
IGA ordinal scale should have approximately 5 severity grades, reported in whole numbers (e.g., 0 to 4). For minimizing interobserver variability, each grade should be defined by a distinct and clinically relevant morphologic description.
-
Severity grade definitions should not include numerical ranges for lesions because IGA scale provide qualitative assessment of subjects condition.
-
The IGA scale decides success or failure, success is defined as clear or almost clear (grade 0 to 1), with at least to 2 grade improvement from baseline, that represents clinically meaningful outcome.
-
No single standardized grading system to check severity of acne, suggestion is to discuss IGA scales and study designs with FDA before implementing trial.
-
Counting of inflammatory and non-inflammatory lesions should be reported separately, and all lesions on face including on nose should be counted.
-
Treatment effect assessment should be based on lesion counts and success on IGA. Endpoints, based on these changes of lesion counts and IGA success, provides quantitative and qualitative assessments of acne, giving useful complementary information.
-
Number of lesion count reduced may indicate improvement of severity, but does not account for variable expression of acne (e.g., size of lesions, intensity of inflammation, and location of lesions).
-
Success or failure of subjects is considered based on IGA with improvement of two grades, but they are not precise measure of magnitude of improvement. Quantitative assessment of improvement is provided by the changes from baseline in lesion counts.
General Analysis Considerations
Statistical analysis address following
-
Lesion count analysis can be influenced by few extreme outliers, which may make in interpretation of the clinical trial findings difficult. Alternative approach for analysing lesion counts if extreme outliers are anticipated (such as analyses based on ranks), to the statistical analysis plan should be considered.
-
Analysis of complete change and percentage change from baseline are both relevant and complementary, for assessing the treatment effect on inflammatory and noninflammatory lesion counts. Absolute change in lesion count will be affected if subjects have large number of lesions at baseline, and percent change will be affected if subjects have relatively small number of lesions at baseline. Absolute change in lesion count will be expected to have better analysis properties, such as less skewed distribution. Therefore, absolute change in lesion count is recommended for primary endpoint analysis, and percent change in lesion count should be considered for secondary endpoint analysis.
Acne Drugs Regulatory amendments and updates:
May 2021 to November 2022: American academy of dermatology has given updated guidelines, for clinical management of patients with Acne Vulgaris. For the evaluation of the safety and efficacy of the acne treatments, approved by United States Food and Drug Administration (FDA), systemic review was performed.
-
Updated guidelines identified evidence of gaps in microbiology use and endocrinology testing, use of systemic antibiotics other than tetracycline class antibiotics, complementary and other therapies, dietary requirements, physical treatments, and cost affordability for the ance treatment.
-
For use of topical therapies, AAD group suggest the use of multimodal therapy combining multiple mechanisms of action to improve effectiveness also lowering the risk of antibiotic resistance.
-
For treating severe acne systemic drugs are frequently used which can cause complications for pregnant women’s, children below age od 9 to 12 years. AAD group has recommended to not use oral antibiotics as a monotherapy and systemic antibiotics should be used in limit, for shortest possible duration, no longer than 3 to 4 months to reduce risk of antibiotic resistence.
-
The AAD group has given advise to clinicians for monitoring patients for depression, anxiety, suicidal ideation/suicidality, and other neuropsychiatric adverse effects. Treatment decisions should be personalized based on individual responses to this drug.
Cosmo Announces Submission of the trials were successful and Winlevi, based on the effect and safety evidence collected in two identical, randomized, placebo-controlled phase III clinical trials, involving >1,400 patients with acne vulgaris. Got susccesful in clinical trials and met the three co-primary endpoints, indicating treatment success in Investigator’s Global Assessment (IGA) and lowered acne inflammatory and non-inflammatory lesion count. Winlevi was approved in 2020 by FDA, as a novel drug having unique mechanism of action for the acne topical treatment in patients with age group 12 years and older.
Acne Drugs Regulatory challenges:
Stringent Regulatory Policies: Stringent regulatory policies are one of the key factors restraining the growth of the global anti-acne treatment market. Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) impose rigorous standards for the approval and commercialization of acne treatment products, including clinical trials, safety assessments, and ingredient testing. For instance, in July 2021, the FDA threatened to fine Accuitis for failing to report the results of a Phase 2 clinical trial for an acne rosacea treatment to the U.S. government registry.
Antibiotic resistance: updated guidelines for acne drug development given by AAD group suggest to make a drug which will be effective for treating acne in very short periods of time, which will lower the risk of developing antibiotic resistance.
Possible Riskin development of Acne Drugs:
Voluntary Product Recall By Market Players: Voluntary product recalls by market players are significantly restraining the growth of the global acne treatment market, as they can lead to several challenges for both consumers and manufacturers. When a company recalls a product, it creates a sense of distrust among consumers regarding the safety and reliability of acne treatments. For instance, in March 2024, acne-treatment products from ma or brands, including proactiv, Target’s Up & Up, and Clinique, have been found to contain elevated levels of benzene, a carcinogenic chemical linked to leukemia. An independent testing laboratory filed a petition with the U.S. Food and Drug Administration (FDA), urging the recall of these products, which all contain the active ingredient benzoyl peroxide. Benzene, a substance naturally found in gasoline and tobacco smoke, is known to pose serious health risks when present in high amounts. The FDA investigated the issue, following the laboratory’s request for a formal recall of the affected acne treatments This recall adds to a growing list of products that have been pulled from the market due to contamination, further heightening consumer awareness of potential health risks associated with everyday personal care items.
Side Effects And Safety Concerns: The global acne treatment market is significantly impacted by the side effects and safety concerns associated with many commonly prescribed medications, such as oral antibiotics, retinoids, and hormonal treatments. These treatments, while effective in managing acne, often come with undesirable side effects, including skin irritation, dryness, and more severe long-term health risks like antibiotic resistance, skin thinning, and hormonal imbalances. For instance, in September 2023, American Academy of Dermatology Association reported that isotretinoin, commonly prescribed for severe acne, can cause several side effects, including dryness of the skin, chapped lips, nosebleeds, dry eyes, and mouth.
Risk mitigation strategies for Acne Drugs:
-
The mitigation section covers the major regulatory shifts which can possibly affect the adoption of a product in a geography, for instance, Us, Canadian, UK NICE, and European guidelines suggest use of lymecycline and doxycycline over minocycline, due to the effectivity of first 2 drugs and considering higher incidence of severe adverse events associated with minocycline. In addition, US FDA approved sarecycline, a narrow spectrum tetracycline drug which was not approved by EMA, sarecycline has ability to specifically target C. acnes and minimizing disruption of other gut microbiome, which in turn reduce the risk of antibiotic resistance.
-
FDA has given guidelines for the clinical trial designs, study design and efficacy endpoints following it properly will help in mitigating most of the risks in development of acne drugs. Selection of patients for the clinical trials must be done very carefully based on acne type. And designs of the clinical trial must be flexible for dosage optimization process and optimal treatments.
Acne Drugs Competitive Landscape Dashboard:
Companies With Marketed Acne Drugs Products:
-
Pfizer Inc.
-
Abbvie, Inc.
-
Bausch Health Companies Inc.
-
Galderma
-
Mayne Pharma
-
Cosette
-
Pharmaceuticals
-
Johnson & Johnson Inc
-
Sun Pharmaceutical Industries, Ltd.
-
F. Hoffmann-La Roche Ltd.
-
GSK PLC
Regulatory Landscape - Table of Content
Table of contents will appear here once available.
Customer Stories

“This is really good guys. Excellent work on a tight deadline. I will continue to use you going forward and recommend you to others. Nice job”

“Thanks. It’s been a pleasure working with you, please use me as reference with any other Intel employees.”

“Thanks for sending the report it gives us a good global view of the Betaïne market.”

“Thank you, this will be very helpful for OQS.”

“We found the report very insightful! we found your research firm very helpful. I'm sending this email to secure our future business.”

“I am very pleased with how market segments have been defined in a relevant way for my purposes (such as "Portable Freezers & refrigerators" and "last-mile"). In general the report is well structured. Thanks very much for your efforts.”

“I have been reading the first document or the study, ,the Global HVAC and FP market report 2021 till 2026. Must say, good info! I have not gone in depth at all parts, but got a good indication of the data inside!”

“We got the report in time, we really thank you for your support in this process. I also thank to all of your team as they did a great job.”