
Regulatory Landscape - Overview
Obesity Drugs Regulatory Landscape: Product Overview
Obesity is one of the major concerning health disease worldwide, caused due to the accumulation of excess fat in the body. Obesity is diagnosis depends on Body Mass Index (BMI (kg/m2)) which is persons weight divided by the square of their height (weight (kg)/height² (m²). obesity is defined by BMI greater than or equal to 30. According to the WHO, 1 person in every 8 people in world was found to be living with obesity in the year 2022.
There are anti-obesity drugs developed to overcome this health issue, following proper regulatory guidelines while developing these drugs is utmost necessary for ensuring the safety and efficacy of these drugs. US FDA has issued guidance “Obesity and Overweight: Developing Drugs and Biological Products for Weight Reduction” for providing recommendatory guidelines to industries regarding development of anti-obesity drugs or biological products which are regulated within (CDER) Centre for Drug Evaluation and Research in the Food and Drug Administration (FDA). The main intension of the issue of these guidelines is the reduction and long-term maintenance of body weight in obesity patients.
Six drugs are approved by FDA for long term use, out of which four drugs are approved for adults and children with age 12 or more. setmelanotide (IMCIVREE), is a approved drug and is limited for only those patients who are diagnosed with one of four specific rare genetic disorders, which can be confirmed by genetic testing.
Obesity Drugs Types
Based on treatment type, the global anti-obesity drugs market has been segmented into Appetite Suppressants, Lipase Inhibitors, GLP-1 Receptor Agonists, combination drugs, and others. Appetite Suppressants is further segmented into Serotonin Norepinephrine Reuptake Inhibitors, Selective Serotonin 2C Receptor Agonists and Norepinephrine-Dopamine Reuptake Inhibitors (NDRIs).
GLP-1 Receptor Agonists
Glucagon-like peptide-1 receptor (GLP-1R) agonists is a group of therapeutics that targets incretin hormone action, and its receptors are widely distributed in nerves, islets, heart, lung, skin, and other organs. GLP-1R agonists show hypoglycemic effects and reduce weight.
GLP-1R agonists are further divided into short acting preparations and long-acting preparations based on the time-effect and the volume of drug.
Form/ ingredients used
Short-acting preparations include beinaglutide (BN) and exenatide (EX), which generally need to be injected 2-3 times a day. Long-acting preparations include lixisenatide (LIXI) and LIR, which are injected once a day. Long-acting preparations also include semaglutide (SMG), dulaglutide (DUL), long-acting release formulation of exenatide (EX-LAR), and polyethylene glycol loxenatide (PEX-168), which generally need to be injected once a week. Semaglutide is further divided into injectable semaglutide and oral semaglutide.
Obesity Drugs Mode of action
Endogenous GLP-1 is a main component of entero-insular axis, GLP-1 receptor agonists simulate the action of endogenous GLP-1. GLP-1 receptors are widely distributed across the main tissues and organs, by binding to them there is a initiation of weight loss and reduction of food intake, which inturn help in overcoming obesity health disease.
Obesity Drugs Applications
Through mechanisms such as appetite suppression, increased satiety, and improved insulin sensitivity, GLP-1 receptor agonists have demonstrated significant efficacy in promoting sustained weight loss, with clinical trials showing reductions of up to 15-20% of body weight in patients.
Their dual benefit of managing obesity and enhancing metabolic health makes them particularly attractive for individuals with obesity-related conditions like type 2 diabetes and cardiovascular risk factors.
orlistat was found to cause a total body weight loss of 2.4% after 4 years. More importantly, it significantly lowered the risk of type 2 diabetes mellitus (T2DM), Orlistat also improved blood pressure (BP), insulin sensitivity, and lipid profiles as a result of its primary action of reduction of intestinal fat absorption.
Some people can loose weight without any medication with the help of diet and exercise, by following this around 3-4% weight can be reduced. others may require medical help or surgical procedures to be able to lose and maintain weight.
Bariatric surgery is said to be the gold standard intervention for managing obesity as it reduces the impact of counter-regulatory hormones such as ghrelin. It helps people to lose an average of 25–30% of their body weight but all p[patients are not willing to undergo surgery therefore medication may be a good alternative.
Currently approved anti-obesity medications (AOM) by the United States of America (USA) Food and Drug Administration (FDA) include liraglutide (Saxenda), naltrexone-bupropion (Contrave), orlistat (Xenical), phentermine-topiramate (Qsymia), semaglutide (Wegovy), setmelanotide (IMCIVREE), and tirzepatide (Mounjaro).
Obesity Drugs Product Development Steps:
FDA Approval Process for anti-obesity Drugs
To speed up the new drug development process, FDA has formed 4 methods which include, Fast Track, Breakthrough Therapy, Accelerated Approval (AA), and Priority Review.
Fast track designation grants for anti-obesity drug by FDA
- Tirzepatide has been granted with fast-track designation based on its promising results of recent Phase III clinical trial called SURMOUNT-1. This designation aims to accelerate the development and review process of new drugs to treat serious conditions and fill an unmet medical need. Tirzepatide is a GLP-1 and GIP receptor agonist which lowers the appetite, lowered rate of food release from the stomach, increased insulin response and glucagon inhibition.
The drug approval and development process will include following major steps:
- Preclinical Research and Development: This step includes 2 important parts, first is research and discovery of new drug formulation and then undertaking animal testing of that novel drug to check its efficacy and safety
- Investigational New Drug Application: If drug show positive results in first step, then pharmaceutical company moves to the next step of submitting that drug for an investigational new drug (IND) application to FDA, this drug submission will include potential and safety details of the drug.
- Clinical Trials: clinical trials include phase 1, 2 and 3, during this phases drug is tested on healthy volunteers and actual patient population, on small and large scale to check the optimum dosage levels, side effects of the drug Phase 1-safety and dosage: This is a small-scale trial on healthy volunteers to check the safety and dosage levels of the drug, this trial in undertaken for several months.
- New Drug Application and Biologics license applications: once drug is successful in clinical trials next it needs to be submitted for NDA and BLA, NDA is for traditional small drug molecules and BLA is for biological products. This submission to FDA is the key step in getting the drug approved.
- Advisory Committee Meetings: An Independent panel of experts meets to recommend drug approval. Their expertise and advise help the FDA make decisions.
- FDA Decision: FDA Makes Decisions Based on Drugs Risks and Benefits, If Approved, Drug Moves to The Next Step of Manufacturing, Labeling, And Distribution Preparations.
- Post Market Surveillance: FDA monitors the drug even after its approval for ensuring drugs safety and efficacy
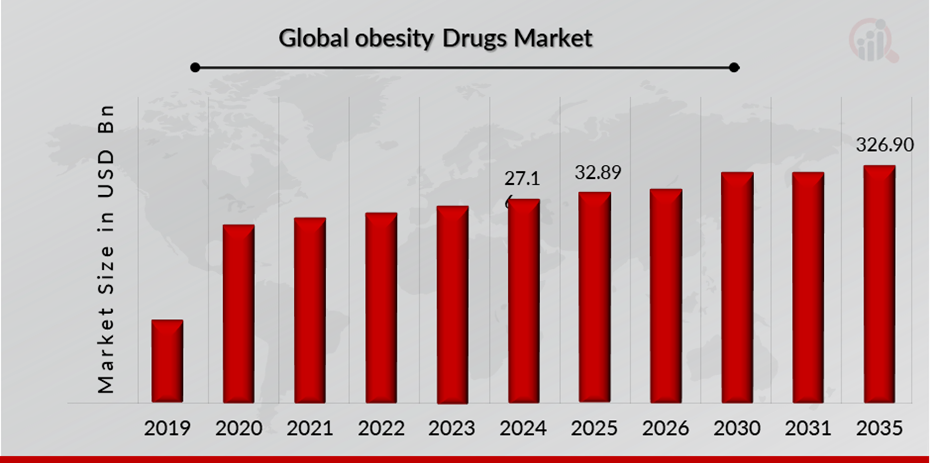
Obesity Drugs Market Size Overview:
As per MRFR analysis, the Obesity Drugs Market Size was estimated at 27.16 (USD Billion) in 2024. The Obesity Drugs Market Industry is expected to grow from 32.89 (USD Billion) in 2025 to 326.90 (USD Billion) till 2035, at a CAGR (growth rate) is expected to be around 25.82% during the forecast period (2025 - 2035). Increased cancer incidence and prevalence and advancements in obesity research and drug development are the key market drivers enhancing the growth of the market.
Source: Secondary Research, Primary Research, MRFR Database and Analyst Review
Obesity Drugs Regulatory Landscape:
There are several key regulatory agencies oversee the approval and monitoring of oncology drugs to ensure their safety, efficacy, and quality.
| Regulatory agencies | Regulatory Ministry |
| Federal Food and Drug Administration | United States: Department of Health and Human Services (HHS) |
| The Medicines and Healthcare products Regulatory Agency | United Kingdom: The Medicines and Healthcare products Regulatory Agency (MHRA) under the Department of Health and Social Care (DHSC) |
| Central Drug Standard Control Organization | India: The Ministry of Health and Family Welfare |
| Health Canada | Canada: The Ministry of Health |
| Pharmaceuticals and Medical Devices Agency (PMDA) | Japan: Ministry of Health, Labour and Welfare. |
| National Medical Products Administration (NMPA) | China: The Ministry of Health |
| Health Sciences Authority | Singapore: The Ministry of Health |
| European Medicine Agency | European union |
| Therapeutic Goods Administration (TGA) | Commonwealth of Australia |
Obesity Drugs Guidelines:
Eligibility: Current guidelines suggest individuals who have body mass index (BMI) of ≥ 30 kg/m2 or ≥ 27 kg/m2 with an obesity-related comorbidity are eligible for weight loss medication treatment.
Obesity Drug Types: obesity drugs are classified as long-term acting and short-term acting drugs.
Dosage: For orlistat 60 or 120 mg TID during or within 1 hour of a fat containing meal is the dosage. As per national institute of diabetes and digestive and kidney diseases, If the obesity patient is not lose at least 5% of the weight at starting after taking 12 weeks of full dose of medication, healthcare professionals may advise to stop or change dose of medication or change the treatment
Classification of the Product:
Obesity Drugs Regulatory Process Overview, By Country:
FDA Approval Process for Obesity Drugs
Clinical Assessment of Weight-Reduction Drugs in Adult Patients
Phase 1 and Phase 2 Trials
For starting with phase 3 clinical trials the pharmacokinetics (PK), pharmacodynamics (PD) and dose responses of new weight reducing drug should be evaluated properly.
In early phase trials study of safety and tolerability of broad range of doses is necessary, in phase 1 trial examining PK and PD profile of a weight-reduction drug across a wide range of BMIs, covering the population which is likely to receive the drug. Also, assessment of interactions of drugs and impact of intrinsic and extrinsic factors on PK and PD of drug in investigation, should be done in early stage of drug development to help in designing the later phase trials.
Identification of the range of doses and appropriate dosing regimens to take in phase 3 trials should be done in phase 2 trials, phase 2 duration will cover time to capture maximum or near maximum weight reduction effects of active dosing regimens, also it should include effect of weight reduction drug dose on common weight-related comorbidities (e.g., type 2 diabetes mellitus, hypertension, dyslipidemia), and this data should be considered by the sponsor for selecting most suitable dosing regimen(s) for phase 3 trials.
Design of trial, its size, and duration should be account for dosing considerations like if dose will be used in fixed dose or dose titration regimen or is there need to improve the tolerability for period of dose escalation to achieve target dose. Adults with BMI greater than or equal to 30 kg/m2 or greater than or equal to 27 kg/m2 should be considered as subjects to be included in phase 2 efficacy and safety study if accompanied by at least one comorbidity.
Primary efficacy endpoint should be comparison between mean % change in the weight of body of the group taken for administering investigational drug and the group taken as a control.
If sponsor wants to use one or more clinical outcome assessments for supporting claims of labelling, sponsor should take FDA input at earliest through the whole process of drug development and ensure inclusion of fit for purpose COA in phase 3 trial. Also, the discussion of endpoints, analyses and anchors for ensuring COA results are meaningful clinically and interpretable should be done by the sponsors with the agency in early development program of drugs.
Phase 3 Clinical Trials
Trial design and patient populations
Phase 3 clinical trials doing the examination of effectiveness and safety of weight reducing drugs Should be randomized, placebo controlled, double blind for investigational drug used as a add on for standard recommendation for diet and physical activity in all of the randomized subjects.
Trials should include subjects with these characteristics for ensuring that trial subjects have or are at major risk for weight related morbidity and mortality:
- BMI greater than or equal to 30 kg/m2, having a representative sample of subjects with Class 3 or severe obesity (BMI greater than 40 kg/m2).
- BMI greater than or equal to 27 kg/m2 in the presence of at least one weight-related
comorbidity (e.g., type 2 diabetes, hypertension, dyslipidemia, sleep apnea, or
cardiovascular disease).
As the observed effect of the treatment by the drug can be sequentially different in all subjects who are taking concomitant glucose-lowering medications, it is reasonable to take one or more trials that include only subjects with diabetes at baseline.
Development program should include subjects having comorbidities, like cardiovascular disease, heart failure, liver disease, and chronic kidney disease. Subjects should reflect patients which are likely to use the drug in clinical practice with regard to age, sex, race, and ethnicity in the U.S. population
Trial Size and Duration
General recommendatory guidelines for sample size for assessment of the safety of weight reduction drug is 3000 subjects randomly selected for the investigational drug trial within to be recommended dosage range and no less than 1500 subjects are randomly selected for placebo for duration of at least 1 year of treatment at the maintenance dosage.
Sponsor developing multiple dosing regimens should take into account randomization scheme which assigns more subjects for higher doses and should discuss overall safety database size with the agency at or before the end of the phase 2 trial. Sample size recommended will be able to provide 80% power to detect, with 95% confidence, and 50% approximately increase in incidence of adverse events that will occur at 3% rate in placebo group (i.e. 4.5% to 3 %).
Sample size recommended will also help for efficacy and safety analyses conducted within important subgroups such as age, sex, race, ethnicity, and baseline BMI, if sufficient number of them are enrolled in each od these groups.
Subject number required for demonstrating efficacy of weight reduction drug in every individual is in general small compared to number required to sufficiently assess safety, sponsors can increase the sample size of two adequate and well controlled trials required to support approval or safety analysis can be done depending on integrated data from multiple adequate and well controlled trials including safety and efficacy studies.
Efficacy Endpoints
- Primary efficacy endpoint
Assessment of weight reduction drug efficiency in adults should be done by analyses of mean % change from baseline weight of body in investigational drug group and control group. And subjects with stable height (adults having achieved terminal height) % change in body weight is equal to % change in BMI.
- Secondary efficacy endpoints
Secondary efficacy endpoints include (not limited), to changes in following metabolic parameters, Blood pressure, Lipoprotein lipids, Fasting glucose, A1C (in subjects with type 2 diabetes)
- Efficacy benchmarks
Generally, a drug is said to be effective in weight reduction and maintenance in patients having obesity or overweight along with comorbidities, only if once a treatment period of 1 year at the maintenance dosage is over and the difference in mean % weight reduction in between the investigational drug and the control group with at least 5% and showing statistically significant difference.
Regulatory amendments and updates for Obesity Drugs:
Payor and State Limitations on Off-Label Prescribing of GLP-1s
- In June 2023, the Washington Post reported Anthem Blue Cross Blue Shield plans sent approximately 150 letters to Prescribers in three states for prescribing Ozempic drug to non diabetic patients. Insurance companies usually cover GLP 1 drugs for approved (on label). If doctors prescribe them for other conditions, it could be seen as fraudulent and can be subjected to serious legal consequences even civil or criminal penalties by state medical board.
- Mississippi banned off label GLP1 use for weight loss, also advised to Compounding pharmacies should ensure they are complying with applicable requirements. Prescribers and pharmacies must follow new rules and regulations and new research.
FDA and State Limitations on Compounding GLP-1s
- Due to the increase in the prescription and compounding of GLP-1 has stimulated state medical and pharmacy boards and FDA s interest
- The example of Alabama board of medical examiners and medical licence commission, which has issued a guidance which states that, some of the physicians’ offices in Alabama were found to be improperly compounding GLP-1 (aka semaglutides).
- The Alabama Medical Board stated to prohibit the use of non-FDA approved active pharmaceutical ingredients or semaglutides salts
- This guidance was published in the wake of FDA communications in 2023 to the National Association of Boards of Pharmacy and Federation of State Medical Boards, and has clarified that drugs approved by the FDA, Wegovy and Ozempic, will continue to be listed on FDA’s drug shortage list. In may 2023 the FDA has also issues alert warning about unsafe compounding practices would meet federal requirements.
Obesity Drugs Regulatory challenges:
- Regulatory agencies like FDA undertake strict clinical trials for ensuring safety and efficacy of the new anti-obesity drugs and since drugs of the treatment of obesity are used for long term effect for weight loss, proving their long-term safety is more challenges.
- There are many combination drugs used for the treatment of obesity, which further increase the regulatory approval trials complexity.
- Finding the most appropriate dosage for long term weight loss maintenance also makes it challenging during pre-clinical ani clinical studies of the drug development and approval process.
Obesity Drugs Possible Risk:
- Side effects and safety issues are a major inhibiter in the global anti-obesity drugs market, preventing mass adoption and sustained usage of anti-obesity drugs. Most anti-obesity drugs have been linked to side effects, both mild and severe, which present a concern to both medical professionals and patients. Most common side effects are nausea, diarrhea, constipation, dry mouth, and dizziness, but more severe complications like tachycardia, hypertension, liver toxicity, and psychiatric side effects like anxiety and depression have also been documented.
- Some weight loss drugs, including sibutramine and rimonabant, were recalled from the market because of cardiovascular risks and serious psychiatric side effects, emphasizing the strict safety requirements placed by regulators like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
- The risk of dependence, abuse, or rebound weight gain following withdrawal also discourages some patients from seeking pharmacological therapy.
Risk mitigation strategies:
- Undertake long term clinical trials to ensure these drugs provide therapeutic efficacy over a long period of time for reducing the weight by the obese patient.
- Do post market surveillance to track the safety data of the drugs to ensure that patients are not suffering with the side effects.
- For development of combination drugs undertake strict clinical testing to make sure that there are no unintended interactions between both the drugs.
- Go for controlled drug distribution, to prevent misuse or off label use of these drugs
Competitive Landscape Dashboard:
Companies With Marketed Obesity Drugs Products:
- Novo Nordisk A/S
- Rhythm Pharmaceuticals, Inc.
- KVK Tech, Inc.
- Currax Pharmaceuticals LLC
- VIVUS LLC
- Eli Lilly and Company
- AstraZeneca
- GSK Plc
- Gelesis
- CHEPLAPHARM
- Arzneimittel GmbH
Regulatory Landscape - Table of Content
Table of contents will appear here once available.
Customer Stories

“This is really good guys. Excellent work on a tight deadline. I will continue to use you going forward and recommend you to others. Nice job”

“Thanks. It’s been a pleasure working with you, please use me as reference with any other Intel employees.”

“Thanks for sending the report it gives us a good global view of the Betaïne market.”

“Thank you, this will be very helpful for OQS.”

“We found the report very insightful! we found your research firm very helpful. I'm sending this email to secure our future business.”

“I am very pleased with how market segments have been defined in a relevant way for my purposes (such as "Portable Freezers & refrigerators" and "last-mile"). In general the report is well structured. Thanks very much for your efforts.”

“I have been reading the first document or the study, ,the Global HVAC and FP market report 2021 till 2026. Must say, good info! I have not gone in depth at all parts, but got a good indication of the data inside!”

“We got the report in time, we really thank you for your support in this process. I also thank to all of your team as they did a great job.”