
Neonatal Intensive Care Units Regulatory Landscape
Regulatory Landscape - Overview
Neonatal intensive care device Regulatory Landscape: Product Overview
Neonatal diseases and disorders are the broad range of spectrum of health conditions, affecting infants or newborn babies, usually babies in first 28 days from birth. Infants can acquire neonatal diseases during pregnancy, labour, or at the time of birth and very soon after the birth. A Neonatal Intensive Care Unit (NICU) is a specialized medical facility designed to provide comprehensive, high-level care for newborns who are critically ill or premature. The NICU is equipped with advanced technology and staffed by a multidisciplinary team of healthcare professionals to address the complex medical needs of neonates.
Under U.S. food and drug administration (FDA), centre for devices and radiological health (CDRH) is responsible for looking after the regulations to ensure quality, safety and efficacy of the medical devices used in neonatal intensive care.
Neonatal intensive care device types
Based on Product, the global neonatal intensive care market has been segmented into Incubator, Warmer, Respiratory Equipment, Phototherapy Equipment, and Surgical and Monitoring Equipment.
| Incubators | Warmers | Respiratory Equipment | Phototherapy Equipment | Surgical Monitoring Equipment |
| Open Incubator Closed Incubator Transport Incubator Warmer-Cum-Incubator (Convertible Unit) |
Radiant Warmers Transport Warmers |
Ventilator CPAP Machines Oxygen Therapy Devices Resuscitator Accessories |
Bili Lights Accessories | C-Arm Machine Defibrillator Anesthesia Machine Cardiac Monitors Pulse Oximeters Temperature Monitors Therapeutic Hypothermia (HIE) Equipment |
Applications of Neonatal intensive care units
Incubator: The rising prevalence of preterm births around the world has significantly increased demand for neonatal incubators. With the increasing preterm births across worldwide, incubators became critical in providing a controlled, sterile, and temperature-regulated environment for premature infants, who are highly susceptible to infections and temperature instability. Furthermore, use of advanced, technologically equipped incubators provides improved monitoring systems, automated temperature regulation, and real-time neonatal health tracking.
Warmer: For immediate stabilization of newborns, particularly those born with low birth weight or who required resuscitation at birth. Neonatal warmers are essential for keeping infants' bodies warm, which is critical for their survival in the first hours after birth. Warmers are commonly used in the delivery room or NICU to prevent newborn hypothermia, particularly in developing countries where healthcare access is often limited.
Respiratory Equipment: Respiratory support for neonates, particularly those with respiratory distress syndrome (RDS), was a long-standing practice prior to COVID-19. Neonates, particularly preterm infants, frequently have respiratory problems due to underdeveloped lungs, so the demand for neonatal respiratory equipment such as ventilators, continuous positive airway pressure (CPAP) devices, and oxygen therapy systems was increasing. Advances in neonatal respiratory equipment, such as non-invasive ventilation solutions, have helped reduce the risk of infection and subsequent lung damage. This was especially important in areas with a high incidence of neonatal respiratory disorders.
Phototherapy Equipment: Neonatal jaundice, which affects a large number of newborns, necessitated widespread use of phototherapy equipment. These units create a safe and controlled environment for treating jaundice by exposing infants to blue light, which helps break down excess bilirubin in the blood. The growing number of neonatal jaundice cases around the world, particularly in areas with limited access to healthcare, has increased demand for phototherapy devices. Furthermore, innovations in phototherapy equipment, such as fiber-optic devices and LED-based technologies, provided safer, more energy-efficient, and portable solutions, which helped to drive market growth.
Surgical and Monitoring Equipment: As neonatal care became more sophisticated, there was a greater emphasis on intensive monitoring and surgical intervention for critically ill newborns. The introduction of integrated monitoring systems, which enabled clinicians to track vital signs like heart rate, oxygen saturation, and blood pressure in real time, was a significant development. Surgical interventions, while less common, increased the demand for advanced neonatal surgical equipment. Many premature or critically ill neonates require surgery to treat congenital abnormalities or prematurity-related complications. Monitoring equipment, such as pulse oximeters, ECG monitors, and multi-parameter monitors, was critical for ensuring neonates' safety during and following surgery.
Neonatal intensive care medical devices Development Steps:
Under CDRH there is office of product evaluation and quality (OPEQ) which implements program for the evaluation of medical devices or clearance for clinical investigation and marketing, like premarket notification, premarket approval application (PMA), investigational device exemptions (IDEs), humanitarian device exemption (HDE), De Novo and Product Development Protocols (PDPs) programs.
Public health is protected and promoted by evaluation, enhancement and ensuring compliance with medical device laws with recalls, inspections, audits, registrations and listings, allegations of regulatory misconduct, import and export, premarket and labelling and bioresearch monitoring programs.
FDA has segmented medical devices into class I, class II, class III. Class I devices are of low risk and need to have general controls like good manufacturing practices, labelling, registration. Mostly they are exempted from premarket notifications.
Class II devices pose moderate risk and need special controls as they pose higher risk than class I devices, requiring additional performance standards, post market surveillance, and labelling requirements, they usually need premarket notifications.
And class III devices are of high risk and need Pre-Market Approval (PMA), They usually include life sustaining, supporting, and long-term implantable devices in their category, which require clinical trials and detailed safety data. So the key market players developing neonatal intensive care medical devises must identify the class their products according to these FDAs guidelines and get required PMA, premarket notification or other general controls.
Neonatal Intensive Care Medical Devices Market Size Overview:
As per MRFR analysis, the Neonatal Intensive Care Medical Devices Market Size was estimated at 10,496.78 (USD Million) in 2024. The Neonatal Intensive Care Medical Devices Market Industry is expected to grow from 11,164.28 (USD Million) in 2025 to 17,008.31 (USD Million) till 2032, at a CAGR (growth rate) is expected to be around 6.22% during the forecast period (2025 - 2032). rising incidence of preterm births, advancements in neonatal care technology are the key market drivers enhancing the growth of the market.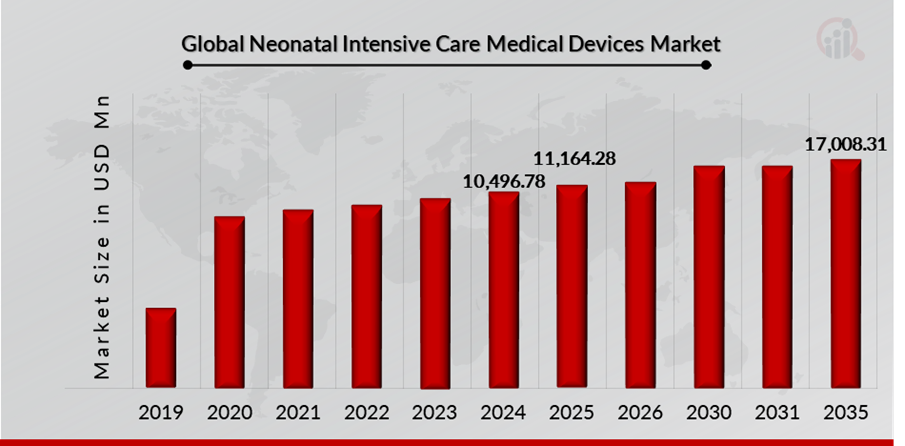
Source: The Secondary Research, Primary Research, MRFR Database and Analyst Review
Catheter Regulatory Landscape:
There are several key regulatory agencies who oversee the approval and monitoring of neonatal intensive care medical devices to ensure their safety, efficacy, and quality.
| Regulatory agencies | Regulatory Ministry |
| Federal Food and Drug Administration | United States: Department of Health and Human Services (HHS) |
| The Medicines and Healthcare products Regulatory Agency | United Kingdom: The Medicines and Healthcare products Regulatory Agency (MHRA) under the Department of Health and Social Care (DHSC) |
| Central Drug Standard Control Organization | India: The Ministry of Health and Family Welfare |
| South African Health Products Regulatory Authority (SAHPRA) | National Department of Health. |
| Pharmaceuticals and Medical Devices Agency (PMDA) | Japan: Ministry of Health, Labour and Welfare. |
| National Medical Products Administration (NMPA) | China: The Ministry of Health |
| Health Sciences Authority | Singapore: The Ministry of Health |
| European Medicine Agency | European union |
| Brazilian Health Regulatory Agency (Anvisa) | Ministry of Health, part of the Brazilian National Health System (SUS) |
Neonatal Intensive Care Medical Devices Guidelines:
Eligibility: For infants or newborn babies, usually in the first 28 days from birth, falling ill, there are 4 classes of NICU based on the requirement of the medical treatment to be given to neonates to treat different health conditions including respiratory distress syndrome (RSD), jaundice, pneumonia, anemia or any other. Below are the classes:
Normal neonatal care level 1
It includes basic newborn care, and having babies with only mild medical conditions like G6PD deficiency, babies born to hepatitis B carrier mothers, and those having mild congenital malformations or born to mothers having maternal complications like diabetes mellitus, pyrexia, prolonged rupture of membrane any many other, babies receiving phototherapy babies, even if they free from all kinds of clinical manifestation of illness are also included in this category.
Special Care Nursery Level 2
Babies of low weight 2000 g and below, or babies with premature deliveries of 35 weeks and below, and those who need continuous monitoring of rates of heart or respiration pulse oximeter, or by transcutaneous monitors, who need extra oxygen, receive intravenous glucose and antibiotics or tube fed, receiving phototherapy, also those who had minor surgery in previous 24 hours, or with persistent hypothermia 360 c and less. Basically, those who need special monitoring.
Following equipment’s are used for their special care; incubator or cot adequate for temperature control, ambient oxygen analyzer, apnoea alarm, heart rate monitor, infusion pump, phototherapy unit access to biochemical analysis using micro methods, access to equipment for radiological examination.
Neonatal Intensive Care Unit Level 3
Under this category babies need continuous monitoring of respiration or rates of heart using pulse oximeter, apnoea monitor, transcutaneous monitors. Includes critically ill babies who need assisted ventilation like Intermittent Mandatory Ventilation (IMV), or Constant Positive Airway Pressure (CPAP), in the first 24 hours and then its withdrawal. Or babies having major medical procedures like arterial catheterisation, peritoneal dialysis or exchange transfusions. Infants having weight less than 1250 grams or premature deliveries below 30 weeks or less should be kept under level 3 neonatal intensive care
Some of the NICU medical devices used for level 3 are as follows intensive care incubator or unit with overhead heating, respiratory or apnoea monitor, heart rate monitor, intravascular blood pressure transducer or surface blood pressure recorder, transcutaneous pO2 monitor or intravascular oxygen transducer, transcutaneous pCO2 monitor, syringe pumps, fusion pumps, ventilator, continuous temperature monitor, pulse oximeter, phototherapy unit, ambient oxygen monitor, facilities for frequent blood gas analyses using micro methods.
Neonatal Intensive Care Medical Devices Classification Of The Product:
Neonatal Intensive Care Medical Devices Regulatory Process Overview, By Country:
Neonatal intensive care medical devices are regulated by U.S. FDA under Center for Devices And Radiological Health (CDRH). There is an office of product evaluation and quality (OPEQ) which evaluates and monitors device performance.
OPEQ includes Office Of Health Technology (OHT) under it which handles various categories of medical devices regulated by FDA, for instance, it has OHT3- Office of Cardiovascular Devices, which regulates cardiovascular support devices, OHT4- Office of Surgical and Infection Control Devices and OHT5- Office of Radiological Health which looks after imaging devices, and many more such categories for particular treatment options and devices used in it.
Regulatory classes are given by FDA for different medical devices which include class I, II, III. Different Neonatal medical devices usually fall under all these categories. And class II need premarket notification 510(K) and class III need premarket approval (PMA) which usually involve clinical trials and detailed safety efficacy data and FDA review process.
The FDA participates in the International Neonatal Consortium External Link Disclaimer (INC), a global collaboration formed to forge a predictable regulatory path for evaluating the safety and effectiveness of medical products for neonates. The consortium has spearheaded many opportunities to improve and accelerate neonatal product development. For example, the INC developed a neonatal adverse event severity scale to help improve the quality of safety evaluations in neonatal clinical trials.
NICU medical devices segmented into different classes are as follows.
| NICU Medical Device | Medical Device Class by FDA |
| Incubators | Class I, II |
| Respiratory Equipment | Class I, II and III |
| Phototherapy Equipment | Class II |
| Surgical and Monitoring Equipment | Class II and III |
Market Player Update:
Product launch: In 2024, Innovative new neonatal product launch by Euruplaz in UK, which intends to save babies’ lives, and has potential to show improvement in neonatal care and save lives more of premature born babies or distress. They have come with an integrated ‘sidestream’ CO2 sampling port, joined to side of existing neonatal flow sensor. This enables clinicians to follow the right protocols of using both lung protective control of volume and monitoring of CO2 which gives best chance of survival for babies and limit possibility of long-term conditions like cerebral palsy or lung disease.
Product recall: In March 2025, recall of Medtronic over neonatal intensive care system which monitors airways of incubated newborns, due to the sensors failing to detect obstructions was done by US FDA. Agency Handed down label of class I for the issue, its most serious designation, spanned 145 affected covidien SonarMed monitors and sensor packages around hundreds of different sizes, distributed in 19 U.S. states.
Neonatal Intensive Care Medical Devices Regulatory Updates and Amendment’s:
January 2025, U.S FDA has provided an update for evaluation of exposure potential to airborne chemicals (formaldehyde, cyclohexanone, and other volatile chemicals) which can be released from neonatal incubators. They have worked with GE HealthCare and implemented a procedure for reduction of the levels of formaldehyde from their new neonatal incubators before distribution.
Further, the FDA has taken actions to continue monitoring the post market performance incubators for neonatal. and inform any significant information or recommendation available to the public.
Neonatal Intensive Care Medical Devices Regulatory Challenges:
Noncompliance with regulatory pathway will lead failure in getting the premarket approval which in turn will delay the approval process.
Material used for medical device development must be biocompatible. Which will not cause harmful effects to newborn babies, failure of it will create safety issues.
Possible Risk in the development of Neonatal Intensive Care Medical Devices:
High Costs of Neonatal Care: One significant restraint in the global neonatal intensive care market is the high cost associated with neonatal care services and advanced medical technologies. The process is complex and involved, bearing large monetary charges for healthcare providers and families. The hospital bill may even be more elevated due to intensive usage of specialized medical equipment, among others through making use of the ventilators and incubators, in addition to needing highly trained health care staff to take care of the critically ill newborn. Prolongation of stay in NICUs may also prolong for the preterm babies. This financial stress is bound to be a significant challenge to most of the families without proper health insurance cover, hence limiting their access to some essential care for vulnerable newborns. Thus, the high cost of neonatal care slows the growth of the market and accessibility to services while threatening to find less costly solutions and increase insurance cover for neonatal care.
Neonatal Intensive Care Medical Devices Competitive Landscape Dashboard:
Companies With Marketed Neonatal Intensive Care Medical Devices
- GE Healthcare
- KoninklijkePhilips N.V.
- Mindray
- Schiller Healthcare India Pvt. Ltd.
- DrägerwerkAG & Co. KGaA
- Nihon Kohden Corporation
- Medtronic
- Shenzhen Comen Medical Instruments Co., Ltd.
- Natus
Regulatory Landscape - Table of Content
Table of contents will appear here once available.
Customer Stories

“This is really good guys. Excellent work on a tight deadline. I will continue to use you going forward and recommend you to others. Nice job”

“Thanks. It’s been a pleasure working with you, please use me as reference with any other Intel employees.”

“Thanks for sending the report it gives us a good global view of the Betaïne market.”

“Thank you, this will be very helpful for OQS.”

“We found the report very insightful! we found your research firm very helpful. I'm sending this email to secure our future business.”

“I am very pleased with how market segments have been defined in a relevant way for my purposes (such as "Portable Freezers & refrigerators" and "last-mile"). In general the report is well structured. Thanks very much for your efforts.”

“I have been reading the first document or the study, ,the Global HVAC and FP market report 2021 till 2026. Must say, good info! I have not gone in depth at all parts, but got a good indication of the data inside!”

“We got the report in time, we really thank you for your support in this process. I also thank to all of your team as they did a great job.”