
Regulatory Landscape - Overview
Veterinary Diseases Drugs Regulatory Landscape: Product Overview
Veterinary diseases is illness in animals including pets, livestock and wildlife, these diseases can be caused due to bacterial, viruses, genetic disorders or any environmental factors. Veterinary medicine is utilised for the prevention, control, diagnosis and treatment of diseases affecting animals. Adherence to the regulations is most important while developing these therapeutics.
FDAs Centre for Veterinary Medicine (CVM) ensures that animal drugs developed are safe and effective, also looks after its packaging and labelling and overall quality of the drugs.
Veterinary therapeutic type
Veterinary therapeutics are segmented into various classes including Tablets, Boluses, Capsules, Feed additives, Drinking water medications, Parenteral dosage form, Oral pastes and gels, Drenches and tubing products, Topical Products and Veterinary vaccines.
Veterinary vaccines
Different types of vaccines are available for various animal diseases. Vaccines used in veterinary disease treatment is segmented into 3 categories: inactivated vaccines (in which antigens are typically combined with adjuvants); attenuated, live vaccines; and recombinant technology vaccines, which may include subunit antigens or genetically engineered organisms.
| Target pathogen | Target animal | Brand name | Distributor | Type of vaccine |
| Lawsonia intracellularis | Pigs | Enterisol Ileitis | Boehringer-Ingelheim Vetmedica | Live oral vaccine |
| Porphyromonas gulae, P. denticanis, and P. salivosa | Dogs | Periovac | Pfizer Animal Health | Killed vaccine against periodontitis |
| Vibrio anguillarum | Fish | AquaVac Vibrio | Schering-Plough Animal Health | Killed oral vaccine |
| Streptococcus equi | Horses | Equilis StrepE | Intervet | Live submucosal vaccine; deletions in aroA gene |
Mode of action
Vaccines insert part of pathogen in the animals, which results in challenging immune system which reacts with the inserted pathogen and create memory cells for antigens of that encountered pathogen. In future when animal encounters same pathogen, immune system recognizes it and generate response immediately before pathogen can cause disease.
For instance, Equilis StrepE is a type of veterinary vaccine utilised for protection of horses against respiratory disease caused by Streptococcus equi bacteria. Equilis StrepE has small amount of bacterium type namely S. equi. When horse is given such a vaccine it is recognized by the bacteria and forms antibodies against it, in future if bacteria again invade, antibodies are quickly produced upon recognition and the infection is prevented. Equilis StrepE is modified by removing certain genes, making it less likely to cause a disease, making it suitable to be used in vaccine.
Applications of veterinary therapeutics
To ensure that animal is free from any kind of pain or suffering, is the main goal of veterinary medicines.
Veterinary vaccines: Infectious disease prevention in animals is most important application of veterinary vaccines. Veterinary vaccines stimulate immune system to make antibodies against specific pathogen to protect it from future infections. In turn it will stop spreading of the diseases, protect animal health.
They will help in increasing production of livestock in cost effective manner. Veterinary vaccines treatment will reduce or prevent the illness and deaths and will increase overall health of the animals which will lead to more productivity and profits to the farmers.
They can be also used for conserving wildlife, by using vaccination for wild animals we can prevent the spread of infectious diseases, for instance, vaccines are used for protecting wild population of raccoons and foxes from rabies.
It even shows influence on public health, prevention and control of animal diseases with veterinary vaccines show direct implications for human health, as some animal diseases are transmitted from animals to humans.
Veterinary drugs: Alternative to vaccines can be veterinary drugs which are primarily used for treatment and management of diseases which includes antibiotics, anti-inflammatory, and antiparasitic. They don’t prevent the disease like the vaccines but only help to control it after it occurs.
List of veterinary drugs approved by FDA
- In February 2025, Tulathromycin Injection was approved by US FDA for the treatment of bovine respiratory disease.
- In January 2025, Eprimectin Pour-On (eprinomectin), approved by FDA, for gastrointestinal roundworms, lungworms, grubs, sucking and biting lice, chorioptic and sarcoptic mange mites, horn flies in beef and dairy cattle.
- In November 2024, BRAVECTO 1-MONTH (fluralaner) was approved by FDA, supplement for treatment and control of Haemaphysalis longicornis (Asian longhorned tick).
veterinary medicine Development Steps:
For veterinary vaccines, Animal Plant Health Inspection Services (APHIS) the U.S. Agriculture Department is responsible for ensuring safety, efficacy and quality of veterinary biologics.
For veterinary Drugs, once determination is done for product to be used as a drug for animal, next process is to determine if it is a new animal drug and sponsors who need to make and sell animal drugs must pass through the NADA process
Veterinary medicines Market Size Overview:
As per MRFR analysis, the Veterinary Medicine Market Size was estimated at 36.20 (USD Billion) in 2024. The Veterinary Medicine Market Industry is expected to grow from 38.23 (USD Billion) in 2025 to 62.54 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 5.62% during the forecast period (2025 - 2034).Growing chronic illnesses, increasing rates of pet ownership, and increased meat and the expansion of the veterinary medicine industry might be fueled by efforts to promote animal husbandry and an increase in pet adoption are the key market drivers enhancing the Veterinary Medicine market growth. 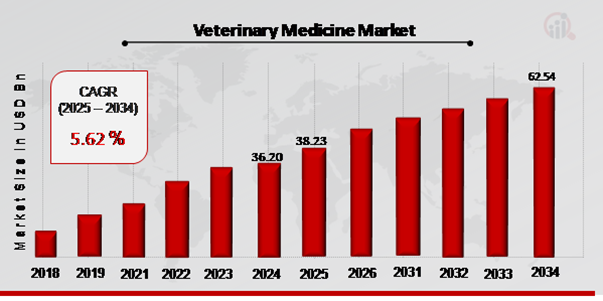
Source: The Secondary Research, Primary Research, MRFR Database and Analyst Review
Veterinary Medicines Regulatory Landscape:
There are several key regulatory agencies that oversee the approval and monitoring of veterinary medicines to ensure their safety, efficacy, and quality.
| Regulatory agencies | Regulatory Ministry |
| Federal Food and Drug Administration- Centre for veterinary medicine (CVM) | United States: Department of Health and Human Services (HHS) |
| Veterinary medicines directorate (VMD) | United Kingdom: The Medicines and Healthcare products Regulatory Agency (MHRA) under the Department of Health and Social Care (DHSC) |
| Central Drug Standard Control Organization- veterinary division | India: The Ministry of Health and Family Welfare |
| South African Health Products Regulatory Authority (SAHPRA) | National Department of Health. |
| Pharmaceuticals and Medical Devices Agency (PMDA)- veterinary medicines section | Japan: Ministry of Health, Labour and Welfare. |
| National Medical Products Administration (NMPA) | China: The Ministry of Health |
| European Medicine Agency- committee for medical products for veterinary use (CVMP) | European union |
| Brazilian Health Regulatory Agency (Anvisa) | Ministry of Health, part of the Brazilian National Health System (SUS) |
Veterinary Medicines Guidelines:
Animals are only prescribed veterinary medications if they are suffering from any illness or disease diagnosed. Based on the type of illness or the animal species they are either prescribed with vaccines or drugs.
For vaccination, based on the time for which the protection is required, the immunization is administered at least twice, and booster doses should be given after every 1 to 2 years, typically after every 3 months. And drugs are administered based on body weight and body surface area of the animal. Large animals, for instance, cows and horses, require dosage which is often measured in mg or g of tablet per kg of body weight of animal. Example of drug prescribed in relatively high doses is sulfonamides; 750 pounds or kg cow will receive 75 grams of medication which is about 15 grams every 150 pounds body weight.
Veterinary Medicines Classification of the Product:
Veterinary Medicines Regulatory Process Overview, By Country:
FFDCA provides U.S. FDA legal authority for approving and regulating drugs for both people and animals. New animal drug is the drug intended for use in animals. And FDA centre for veterinary medicine provides approval and regulates new animal drug.
CVM is having 6 offices working together for approval of new animal drug and post market surveillance. The office of new drug evaluation (ONADE) is preapproval office which is a lead office for reviewal process of the information about new animal drug before its approval.
Under FFDCA act, legal introduction of new drug into interstate commerce is only accepted when it is subjected to either of these;
- New Animal Drug Application (NADA) or abbreviated new drug application (ANADA) under 512 section of the act.
- Conditional Approval (CNADA) under 517 section of the act.
- Emergency use authorization under 564 section of the act.
- Investigational exemption under 512(j) section of the act.
- listing on the Legally Marketed Unapproved New Animal Drug Index for Minor Species (the Index) under section 572 of the Act.
Approval of NADA by CVM indicates that the animal drug is safe, effective when used according to label, it also ensures that strength, purity and quality of the drug is kept constant throughout the batches, furthermore, labelling of drug is ensured to be truthful, not misleading and complete.
In NADA process centre focuses on 2 other factors which include:
- Impact of drug on the environment
- Ensuring safety of the people who administer this drug to the animals
Veterinary Medicines Regulatory Updates and Amendment’s:
The Animal Drug Availability Act (H.R. 2508) (“ADAA”), signed by the President on October 9, 1996, amended the Federal Food, Drug, and Cosmetic Act to provide new flexibility to the way FDA regulates new animal drugs and medicated feeds.
The law was intended to increase the number of approved new animal drugs on the market and was supported by FDA's Center for Veterinary Medicine and a coalition of animal industry groups which included manufacturers of animal health products, veterinarians, and livestock producers. ADAA made changes that were intended to benefit the animal health industry and the nation's animals without compromising FDA's mission to protect the public health. The changes introduced by ADAA include:
amended the definition of substantial evidence of effectiveness, making the process FDA uses to evaluate and approve new animal drugs more flexible. This change eliminated the requirement for field studies and expanded the types of studies that FDA could consider in support of a finding of substantial evidence of effectiveness.
provided for more interaction between animal drug sponsors and the FDA during the drug development process. The law requires FDA to grant a presubmission conference, if requested by the sponsor, to discuss the drug development at its earliest stages. The purpose of a presubmission conference is for FDA and the sponsor to agree on the data needed to establish safety and effectiveness, and the types of studies that can be conducted to generate such data.
provided a procedure for establishing import tolerances for residues of new animal drugs not approved or conditionally approved for use in the United States but lawfully used in other countries and present in imported, animal-derived food and food products. Import tolerances provide a basis for importing and legally marketing such animal-derived food and food products in the United States.
created a new category of drugs, "Veterinary Feed Directive Drugs," that allows the approval and use of new animal drugs in animal feed, on a veterinarian's order.
permitted a range of acceptable/recommended doses to appear on new animal drug product labeling, rather than one optimum dose.
In addition, the law gives direction to FDA on how to review supplements that seek to add a minor use or minor species indication to an approved application.
Veterinary Medicines Regulatory Challenges:
Diverse and Evolving Regulatory Requirements
Veterinary regulations differ across regions, making the process of compliance a challenge for multinational companies. Under U.S. FDA, centre for veterinary medicine (CVM) looks after veterinary drugs regulation process, and in EU, EMA has set the guidelines of veterinary drug regulation, and they have differences which leads to; Longer approval time, due to varying documents requirement, Inefficiencies in regulations, as companies has to tailor submission for every market, Limited access to international market, even after the efforts in harmonization, through organisations like International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VICH), Even after alignment facilitated by VICH, some inconsistencies data expectations and dossier formats create barriers in registrations of global products.
Complex Approval Pathways for Veterinary Medicinal Products (VMPs)
Veterinary medical products approval needs to collect extensive data for safety, efficacy and quality. But it can be lengthy process and even resource intensive, delaying the time to market entry of innovative products. Complex preclinical and clinical studies and strict GMP requirements lead to these delays.
Antimicrobial Resistance (AMR) and Restrictive Regulations
Even for veterinary medicines, the antimicrobial resistance is global health crisis. Overuse or misuse of antibiotics in animals leads to antimicrobial resistance, which makes the regulators in world to implement strict usage control on antibiotics in animals.
Possible Risk in development of Veterinary Medicines:
Regulation of Novel Veterinary Therapies
With the invention and development of new therapies, including gene therapies, precision medicine comes the uncertainties in regulations. Traditional regulatory rules are not tailored as these unique therapies are developed.
Supply Chain and Compliance Challenges
Ensuring safe veterinary medicines supply chain is challenging. Issues like fake drugs. Unauthorised trade and meeting good distribution standards, maintaining traceability, and authenticity in worldwide markets needs strict regulations and latest technological solutions. These supply chain challenges can cause complexity in ensuring process for safety and efficacy of products reaching to end user.
Veterinary Medicines Competitive Landscape Dashboard:
Companies With Marketed Veterinary Medicines Products
- Boehringer Ingelheim
- Ceva
- Chanelle Pharma Group
- Dechra Pharmaceuticals plc
- Elanco
- Merck Animal Health
- Norbrook Laboratories
- Bayer AG
Regulatory Landscape - Table of Content
Table of contents will appear here once available.
Customer Stories

“This is really good guys. Excellent work on a tight deadline. I will continue to use you going forward and recommend you to others. Nice job”

“Thanks. It’s been a pleasure working with you, please use me as reference with any other Intel employees.”

“Thanks for sending the report it gives us a good global view of the Betaïne market.”

“Thank you, this will be very helpful for OQS.”

“We found the report very insightful! we found your research firm very helpful. I'm sending this email to secure our future business.”

“I am very pleased with how market segments have been defined in a relevant way for my purposes (such as "Portable Freezers & refrigerators" and "last-mile"). In general the report is well structured. Thanks very much for your efforts.”

“I have been reading the first document or the study, ,the Global HVAC and FP market report 2021 till 2026. Must say, good info! I have not gone in depth at all parts, but got a good indication of the data inside!”

“We got the report in time, we really thank you for your support in this process. I also thank to all of your team as they did a great job.”