
Regulatory Landscape - Overview
Opioids Regulatory Landscape: Product Overview
Opioids are narcotic analgesics, belonging to class of medicines used to give relief from moderate or chronic pain and also has a sedative effect. They are prescribed by healthcare practitioners for treatment of post-surgery pain, chronic pain, chronic diarrhea, and severe coughing among others.
Centre for drug evaluation and research (CDER) under food and drug administration (FDA) is responsible for the regulation of opioids drugs for ensuring the safety, efficacy and quality of drugs used for different treatment procedures.
Opioids types
Opioids are classified into natural opioids, semi synthetic opioids, or fully synthetic opioids.
| Natural opioids | Semi synthetic opioids | Fully synthetic opioids |
| Codeine | Heroin | Fentanyl |
| Morphine | Hydrocodone | Methadone |
| Opium | Oxycodone | Tramadol |
-
Natural opioids are extracted from seed pods of poppy plant Papaver somniferum.
-
Semi synthetic opioids are synthesized by scientists in lab from codeine or morphine, are more potent than plant-based drugs.
-
Synthetic opioids are completely developed in lab, are more effective than natural and synthetic opioids.
Opioids mode of action
Opioid medicines travel through the blood and attach to opioid receptors in brain cells. This blocks pain messages and can boost feelings of pleasure by blocking the release of neurotransmitters from the neuron ending.
Opioid receptors are proteins, known as G protein-coupled receptors, these are three types of opioid receptors namely, mu, kappa and delta. ‘Mu’ receptors are involved in relieving pain and pleasurable effects of opioids.
They are used in many medications, as they contain chemicals which causes body to relax and relieve pain. Prescription opioids are used mostly for treatment of moderate to severe pain, some of them are used for treating coughing and diarrhea, cough suppression.
Certain are used in anesthesia procedure in medical treatment during surgical procedures to alleviate pain and induce sedation.
FDA has approved these opioids with Labeling describing abuse-deterrent:
| Opioids Drugs approved by FDA | Company |
| OxyContin | PURDUE PHARMA LP |
| Hysingla ER | PURDUE PHARMA LP |
| Xtampza ER | COLLEGIUM PHARM INC |
| RoxyBond | PROTEGA PHARMS |
Opioids Product development
The Division of Anesthesia, Addiction Medicine and Pain Medicine (DAAP) under Office of Neuroscience of centre for drug evaluation and research (CDER) of FDA is responsible for regulation and reviews of Investigational New Drug (IND) applications and marketing of drug and biologic products involved in the treatment of Acute Pain, like Surgical and procedural pain, pain due to trauma/injury, pain due to acute inflammatory processes, then Chronic pain, like cancer pain, neuropathic pain, fibromyalgia, osteoarthritis pain, low back pain and addiction, e.g.: nicotine, alcohol, stimulants, opioids.
They are also involved in regulation of the drug product classes, used in surgical, procedural or ICU settings, providing patient with comfort and/or ease of treatment, e.g.: general anesthetics, local anesthetics, dental anesthetics, topical anesthetics, neuromuscular-blocking agents and neuromuscular-blocker reversal agents.
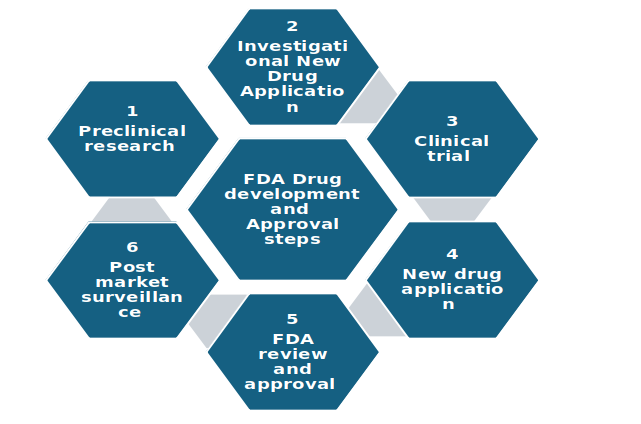
Fig: Development and Regulatory Process Overview for Opioid Drugs.
Opioids Market Size Overview:
As per MRFR analysis, the Opioids Market Size was estimated at 37.47 (USD Billion) in 2022. The Opioids Market Industry is expected to grow from 38.55 (USD Billion) in 2023 to 49.8 (USD Billion) by 2032. The Opioids Market CAGR (growth rate) is expected to be around 2.89% during the forecast period (2024 - 2032). Rising Prevalence of Chronic Pain Conditions, Increasing Investment in Pharmaceutical Research and Development, Regulatory Approvals for Opioid Products are the key market drivers enhancing the growth of the market.
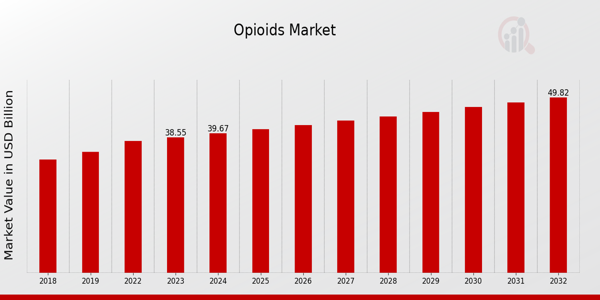
Source: Secondary Research, Primary Research, MRFR Database and Analyst Review
Opioids Regulatory Landscape:
There are several key regulatory agencies who oversee the approval and monitoring of Opioids to ensure their safety, efficacy, and quality.
| Regulatory agencies | Regulatory Ministry |
| Federal Food and Drug Administration | United States: Department of Health and Human Services (HHS) |
| The Medicines and Healthcare products Regulatory Agency | United Kingdom: The Medicines and Healthcare products Regulatory Agency (MHRA) under the Department of Health and Social Care (DHSC) |
| Central Drug Standard Control Organization | India: The Ministry of Health and Family Welfare |
| Health Canada | Canada: The Ministry of Health |
| Pharmaceuticals and Medical Devices Agency (PMDA) | Japan: Ministry of Health, Labour and Welfare. |
| National Medical Products Administration (NMPA) | China: The Ministry of Health |
| Health Sciences Authority | Singapore: The Ministry of Health |
| European Medicine Agency | European union |
| Therapeutic Goods Administration (TGA) | Commonwealth of Australia |
Opioids Guidelines:
Eligibility: Patients suffering with severe to chronic pain due to cancer, postoperative pain, severe injuries, end stage organ disease, neuropathic pain which don’t respond to other treatments are administered with prescription opioids to alleviate the pain.
But if women in pregnancy uses opioids drugs the baby may show the dependence and withdrawal symptoms after the birth., which is called as neonatal abstinence syndrome, use during pregnancy can also lead to miscarriage and low birth weight.
Repeated misuse of prescription opioids can lead to a substance use disorder (SUD), which can develop due to continued misuse of the drug, causing changes in the brain and health problem, which can lead to failure in meeting responsibilities at work, school, or home.
Opioids Classification of the Product:
Opioids Regulatory Process Overview, By Country:
U.S. Food and Drug Administration (FDA) has a main role, in regulating the opioid drugs. They involved in approving new drugs and reformulations, ensuring the safety and efficacy of drugs, and also, along with the U.S. Drug Enforcement Administration (DEA), are helping to monitor the use of available opioid products.
Development of drugs starts with the identifying cellular targets and corresponding candidate compounds.
Important regulatory steps for opioids medication development include as follows:
Preclinical research – laboratory and animal studies assess safety and biological activity of the compound, to determine if drug is safe to take for human trials. Preclinical studies include in vitro or in vivo studies, which establish initial pharmacologic activity and potential for toxicity.
Investigational New Drug Application- IND is submitted before conducting clinical trials, it includes data from preclinical studies, manufacturing information and clinical trial design.
The FDA reviews the IND application, that goes into effect 30 days after submission, if FDA don’t imposes a clinical hold. Once the IND is successful, drug moves for clinical studies.
Clinical trial – includes phase 1 to phase 3
-
Phase 1- test safety and efficacy and dosage in small healthy volunteers’ group. Evaluation of pharmacokinetic and pharmacodynamic parameters is done in this step.
-
Phase 2- Assesses efficacy and side effects in large patients’ group. Includes evaluation of drug's optimal dosage in patients.
-
Phase 3- monitor adverse events if occur, ensure effectiveness in large population of patients. This step requires years to complete.
New drug application – NDA is submitted, which contains all the preclinical and clinical study data, information related to proposed labels, safety updates, and directions for use.
FDA ensures that all the necessary information is submitted within the NDA and needs 60 days review period. The drug is reviewed under a standard 10-month pathway; but, drugs that appear to represent therapeutic advances may be granted a 6-month priority review schedule.
FDA review and approval – FDA team evaluate NDA, consultation from advisory committees with expertise in chemistry and manufacturing, pharmacology, toxicology, statistics, clinical medicine, and any other relevant fields to determine whether the data show that the drug is safe and that there is substantial evidence of its effectiveness. is taken and decision to approve or request more information or deny approval is taken.
Post market surveillance -monitoring drug after launch in market to ensure its safety and effectiveness in general population.
Specific regulations related to opioids
Assessment of abuse potential – when NDA is submitted FDA evaluates the potential of drug abuse this involves analysing factors like drugs pharmacological effects, current scientific knowledge, history and pattern of abuse, public health risks and potential for dependence.
Scheduling recommendations – based on abuse potential assessment the FDA recommends whether drug should be controlled under controlled substances act (CSA) and suggest appropriate schedule I-V. this recommendation is sent to drug enforcement administration (DEA) which makes final scheduling determinations.
4 schedules under CSA act are as follows
-
Schedule 1 – contains drugs of highest abuse potential and no medical use.
-
Schedule 2- drugs has high abuse potential and some medical use.
-
Schedule 3- these drugs have moderate abuse potential
-
Schedule 4- contains drugs with low abuse potential.
-
Schedule 5- drugs with lowest abuse potential.
Opioids are primarily found in schedule 2 to 4 of CSA act.
DEA publish this information of updated list of substances classified under each schedule annually in code of federations regulations (CFR).
Risk evaluation and mitigation – FDA suggest implementation of REMS for certain opioids for ensuring benefits of drug outweigh its risks, REMS program may include elements like medication guidelines, communication plans, elements to assure safe use.
Labelling and prescribing information- FDA ensure all opioid products are properly labelled containing information on indications, usage, dosage, warnings, precautions. This will guide healthcare professionals for safe prescribing opioids to their patients.
Post market surveillance - Development of National Addictions Vigilance Intervention and Prevention Program (NAVIPPRO) was done to provide post-marketing surveillance, signal detection, signal verification, and prevention and intervention programs for scheduled therapeutics.
Other steps like update of shared list for requirements of post marketing was taken by FDA which assess the known serious risks of misuse, OUD, overdose, and death associated with these products, as well as one clinical trial to assess the risk of hyperalgesia associated with long-term, high-dose opioid therapy, for all opioid analgesics.
FDA update on Opioids
December 2023, The FDA has approved new safety Labeling changes for opioid pain medicines. These updates include warnings about the increased risk of overdose with higher dosages, recommendations for the limited use of immediate-release opioids, and a new warning about opioid-induced hyperalgesia (OIH), a condition where opioid use increases pain sensitivity.
October 2024, The FDA has approved a modification to the Opioid Analgesic Risk Evaluation and Mitigation Strategy (OA REMS). This change requires companies to provide pre-paid drug mail-back envelopes to outpatient pharmacies and other dispensers of opioid analgesics by March 31, 2025. The modification also includes updates to the Patient Guide and a new Patient Education Sheet about the risks of unused opioids and the importance of safe disposal.
February 2025, Indian authorities have banned the manufacture and export of two highly addictive opioids, tapentadol and carisoprodol. The investigation revealed that these drugs were being illegally exported by Aveo Pharmaceuticals to West African countries, causing a public health crisis. The Drugs Controller General of India, Dr. Rajeev Singh Raghuvanshi, cited the potential for drug abuse and its harmful impact on the population as reasons for the ban. The FDA has raided Aveo's factory, seized its stock, and halted further production. Further legal action is planned against the company.
Opioids regulatory challenges
December 2023, United Nations International Narcotics Control Board, issued health and regulatory challenges posed by synthetic opioids and non-medical use of synthetic drugs, which includes.
-
Challenges in regulating new synthetic opioids having no known medical use.
-
In developing countries over prescription and non-rational prescription are problems, even they face restricted access certain opioids used in certain pain treatment.
-
Growing concern of exploitation of legitimate manufacturing, marketing, movement, and monetization sectors.
In general, challenges for regulation of opioids drugs include, strict FDA and global regulation, as regulatory bodies impose strict approval process and compliance requirements, delaying product launch.
Risk in development of Opioids
Opioids drugs addiction and side effects are major risk factors setting back its market. They may cause gastrointestinal problems for some patients making them suffer with constipation, nausea, vomiting any many more side effects. Patients body can become tolerant to drug, may feel like taking more drug to get same effect for relieving pain, which can cause drowsiness, slow reflexes, and difficulty in focusing. To overcome these challenges due to opioids people are preferring non opioids treatment products which can hinder the opioids market.
Cannabis has been legalized as an opioid substitute which can limit the expansion of opioids market. Experts are discovering more potential in health advantages which can amid opioid crises. According to one of the survey cannabis use in medical treatment is more viable and alternative approach for replacing highly addictive opioids mostly use for managing mild to severe pain in different medical treatments, constraining market expansion.
Opioids Competitive Landscape Dashboard:
Companies With Marketed Opioids:
-
Sanofi
-
Eli Lilly and Company
-
AbbVie
-
Teva Pharmaceutical Industries
-
Boehringer Ingelheim
-
Hikma Pharmaceuticals
-
Endo International
-
Amgen
-
Mallinckrodt Pharmaceuticals
-
Mylan
-
Johnson and Johnson
-
Pfizer
-
Purdue Pharma
-
Novartis
Regulatory Landscape - Table of Content
Table of contents will appear here once available.
Customer Stories

“This is really good guys. Excellent work on a tight deadline. I will continue to use you going forward and recommend you to others. Nice job”

“Thanks. It’s been a pleasure working with you, please use me as reference with any other Intel employees.”

“Thanks for sending the report it gives us a good global view of the Betaïne market.”

“Thank you, this will be very helpful for OQS.”

“We found the report very insightful! we found your research firm very helpful. I'm sending this email to secure our future business.”

“I am very pleased with how market segments have been defined in a relevant way for my purposes (such as "Portable Freezers & refrigerators" and "last-mile"). In general the report is well structured. Thanks very much for your efforts.”

“I have been reading the first document or the study, ,the Global HVAC and FP market report 2021 till 2026. Must say, good info! I have not gone in depth at all parts, but got a good indication of the data inside!”

“We got the report in time, we really thank you for your support in this process. I also thank to all of your team as they did a great job.”