Increasing Awareness of Product Safety
There is a growing awareness among consumers and healthcare professionals regarding the importance of product safety, which is significantly impacting the Pyrogen Testing Market. As patients become more informed about the potential risks associated with medical products, manufacturers are compelled to prioritize safety measures, including thorough pyrogen testing. This heightened awareness is leading to increased scrutiny of product labels and testing certifications, thereby driving demand for reliable pyrogen testing solutions. In 2025, the market is expected to witness a surge in investments aimed at enhancing testing capabilities, as companies strive to meet consumer expectations. Consequently, the Pyrogen Testing Market is likely to benefit from this trend, as safety becomes a central focus in product development.
Regulatory Compliance and Quality Assurance
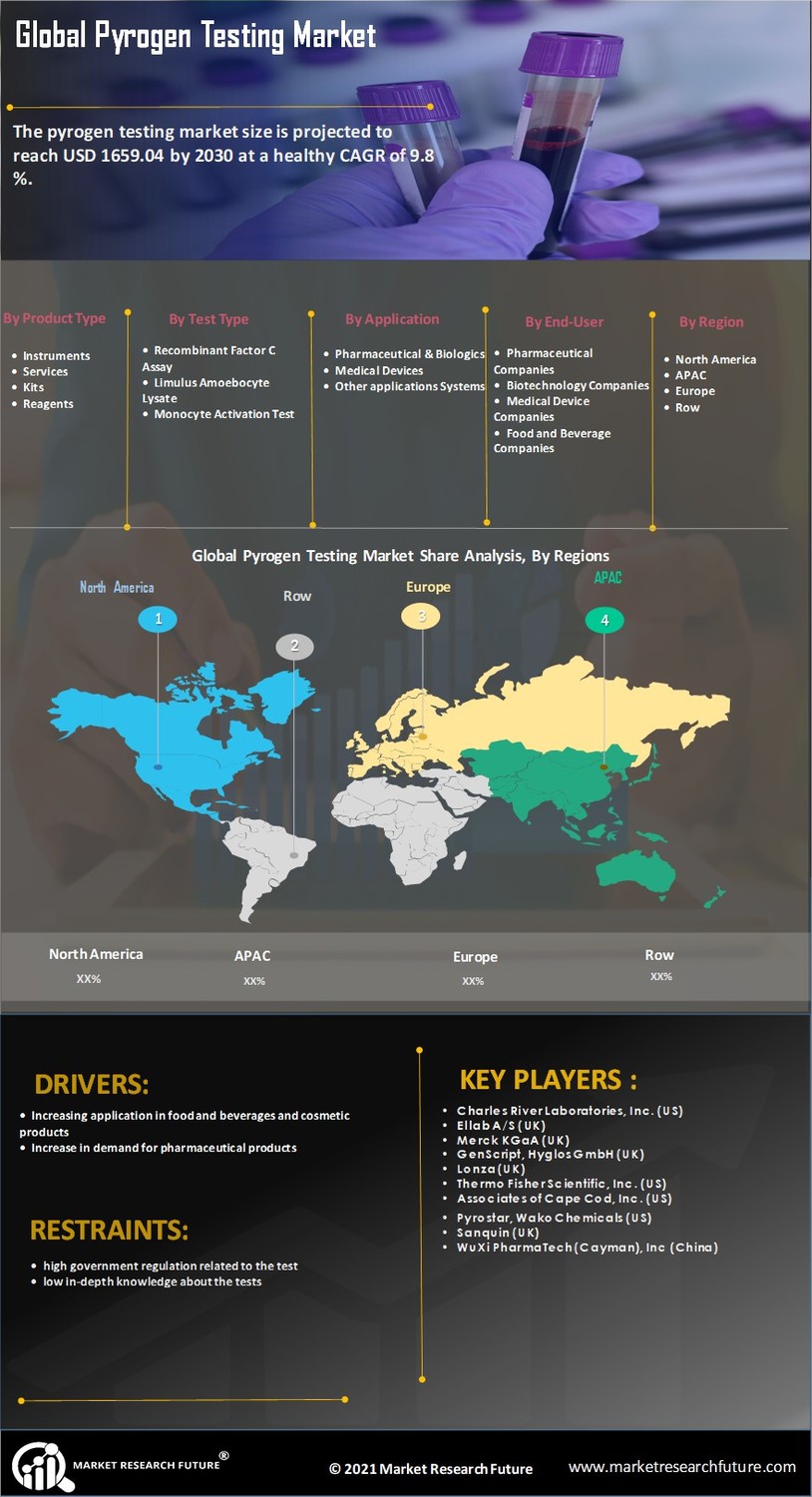
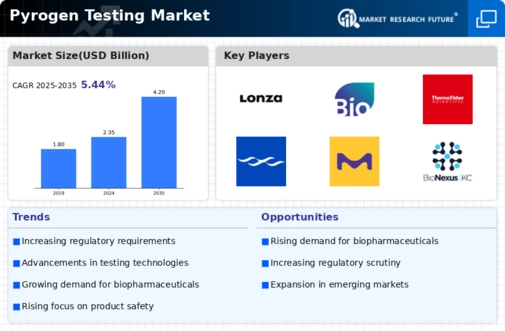
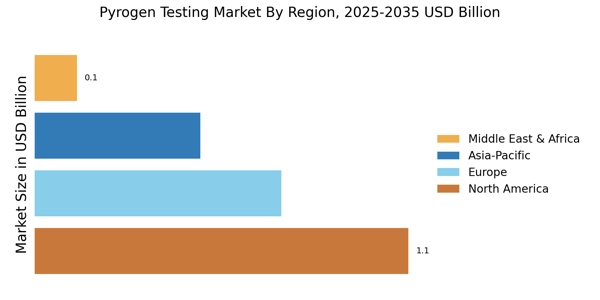
The Pyrogen Testing Market is experiencing a surge in demand due to stringent regulatory requirements imposed by health authorities. Regulatory bodies, such as the FDA and EMA, mandate rigorous testing for pyrogens in pharmaceutical and medical device manufacturing. This compliance ensures that products are safe for human use, thereby driving the need for effective pyrogen testing methods. The market is projected to grow as companies invest in advanced testing technologies to meet these regulations. In 2023, the market for pyrogen testing reached an estimated value of USD 1.2 billion, reflecting a compound annual growth rate of approximately 8% over the past five years. As regulations evolve, the Pyrogen Testing Market is likely to expand further, necessitating continuous innovation in testing methodologies.
Technological Advancements in Testing Methods
Technological advancements are playing a pivotal role in shaping the Pyrogen Testing Market. Innovations such as the development of recombinant Factor C assays and automated testing systems are enhancing the accuracy and efficiency of pyrogen detection. These advancements not only reduce testing time but also improve the reliability of results, which is crucial for manufacturers aiming to comply with regulatory standards. The introduction of rapid testing methods is expected to capture a significant share of the market, as they allow for quicker product release cycles. In 2024, the market for advanced pyrogen testing technologies is anticipated to reach USD 1.5 billion, driven by the increasing adoption of these innovative solutions. Consequently, the Pyrogen Testing Market is likely to witness a paradigm shift towards more sophisticated testing methodologies.
Rising Incidence of Infections and Contaminations
The rising incidence of infections and contaminations in healthcare settings is a critical driver for the Pyrogen Testing Market. As healthcare-associated infections continue to pose significant risks to patient safety, the need for effective pyrogen testing becomes increasingly vital. Contaminated medical devices and pharmaceuticals can lead to severe adverse reactions, underscoring the necessity for rigorous testing protocols. The market is projected to grow as healthcare facilities and manufacturers recognize the importance of implementing comprehensive pyrogen testing to mitigate these risks. In 2025, the Pyrogen Testing Market is expected to expand, driven by the increasing focus on infection control and prevention strategies. This trend highlights the essential role of pyrogen testing in ensuring the safety and efficacy of medical products.
Growth in Biopharmaceuticals and Personalized Medicine
The rise of biopharmaceuticals and personalized medicine is significantly influencing the Pyrogen Testing Market. As the biopharmaceutical sector expands, the need for rigorous pyrogen testing becomes paramount to ensure patient safety. Biopharmaceutical products, including monoclonal antibodies and vaccines, require stringent testing protocols to detect pyrogens effectively. The market for biopharmaceuticals is projected to grow at a rate of 10% annually, further propelling the demand for pyrogen testing solutions. Additionally, personalized medicine, which tailors treatments to individual patients, necessitates precise testing to avoid adverse reactions. This trend is expected to drive the Pyrogen Testing Market towards more specialized and targeted testing approaches, thereby enhancing overall product safety and efficacy.