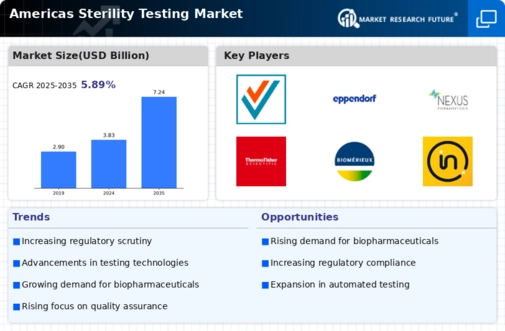
The Americas Sterility Testing Market is experiencing significant growth due to various key market drivers. Increasing regulations and quality standards in the pharmaceutical and biotechnology sectors are propelling the demand for sterility testing. Companies are focusing more on ensuring product safety and compliance, which is essential for preventing contamination and ensuring the efficacy of their products. The rising prevalence of infectious diseases is also contributing to the growth of this market as more companies invest in the development of sterile products. Additionally, the growing biopharmaceutical industry is fostering innovation in sterility testing methodologies, driving market expansion further.
Opportunities available in the market are quite substantial, especially with the increased inclination towards custom-made medicine and advanced drug delivery systems. The move towards biologics and biosimilars creates an even higher demand for trustworthy sterility testing, which opens a door for companies to devise and modify existing testing approaches. Through collaborations and partnerships, for example, testing laboratories and manufacturers can improve their service range and increase efficiency. The focus on automation in testing processes also provides a way for advancement as companies aim to lessen human error and increase the strengths of their tests.
The recent trends suggest that the focus is shifting to more reliable but fast-acting methods of sterility testing for results without losing accuracy. There is also a growing appetite for molecular diagnostics and rapid microbiological testing, among others, which are progressively becoming the norm within the industry. Applications of automation and robotics in testing procedures are on the rise and improving productivity and reliability of results in the sterility testing department.
In addition, there is an ever-growing change in the regulatory environment, which compels businesses to implement more stringent and far-reaching protocols of sterility testing so as to remain compliant and competitive in this rapidly changing global market.
Source: Primary Research, Secondary Research, Market Research Future Database and Analyst Review