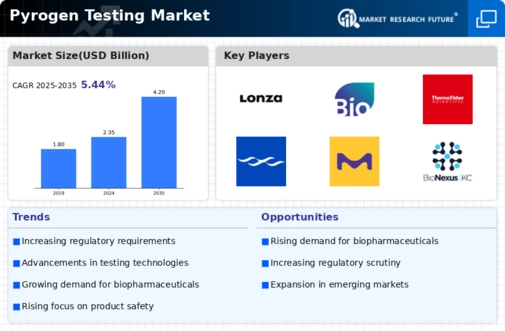
Top Industry Leaders in the Pyrogen Testing Market

July 2023: Charles River Laboratories acquires Toxikon Corporation, expanding its endotoxin testing capabilities and global reach in the pharmaceutical industry.May 2023: Lonza forms a strategic partnership with MicroSafe to offer integrated endotoxin testing and microbial identification solutions for biopharmaceutical manufacturers.October 2023: Thermo Fisher Scientific launches their Endosafe-Nexgen® II system, featuring improved automation and throughput for high-volume endotoxin testing in pharmaceutical labs.August 2023: Sartorius Stedim Biotech introduces their new PyroTec™ P Plus instrument, offering enhanced sensitivity and flexibility for endotoxin testing of medical devices.
- Charles River Laboratories Inc. (US)
- Ellab A/S (UK)
- Merck KGaA (UK)
- GenScript
- Hyglos GmbH (UK)
- Lonza (UK)
- Thermo Fisher Scientific Inc. (US)
- Associates of Cape Cod Inc. (US)
- Pyrostar
- Wako Chemicals (US)
- Sanquin (UK)
- WuXi PharmaTech (Cayman)
- Inc (China)