Advancements in Biotechnology
Advancements in biotechnology are playing a pivotal role in shaping the Preclinical CRO Market. The emergence of innovative biopharmaceuticals, including monoclonal antibodies and gene therapies, necessitates sophisticated preclinical testing methodologies. As biotechnology continues to evolve, preclinical CROs are adapting by incorporating cutting-edge technologies such as CRISPR and high-throughput screening into their service offerings. This integration not only enhances the accuracy and efficiency of preclinical studies but also aligns with the increasing complexity of modern drug candidates. The market for biopharmaceuticals is projected to grow significantly, potentially exceeding 600 billion USD by 2025, further driving the demand for specialized preclinical services. Consequently, preclinical CROs that can effectively leverage these advancements are likely to gain a competitive edge in the industry.
Regulatory Landscape Evolution
The evolving regulatory landscape is significantly influencing the Preclinical CRO Market. Regulatory agencies are continuously updating guidelines to ensure the safety and efficacy of new drugs, which in turn affects the preclinical testing requirements. As regulations become more stringent, pharmaceutical companies are increasingly turning to preclinical CROs to ensure compliance with these evolving standards. This trend is particularly evident in the areas of toxicology and pharmacology, where rigorous testing protocols are mandated. The need for expertise in navigating these complex regulatory frameworks is driving demand for preclinical services. Furthermore, as regulatory bodies emphasize the importance of data integrity and transparency, preclinical CROs that can demonstrate compliance and provide robust documentation are likely to thrive in this competitive environment.
Rising Demand for Drug Development
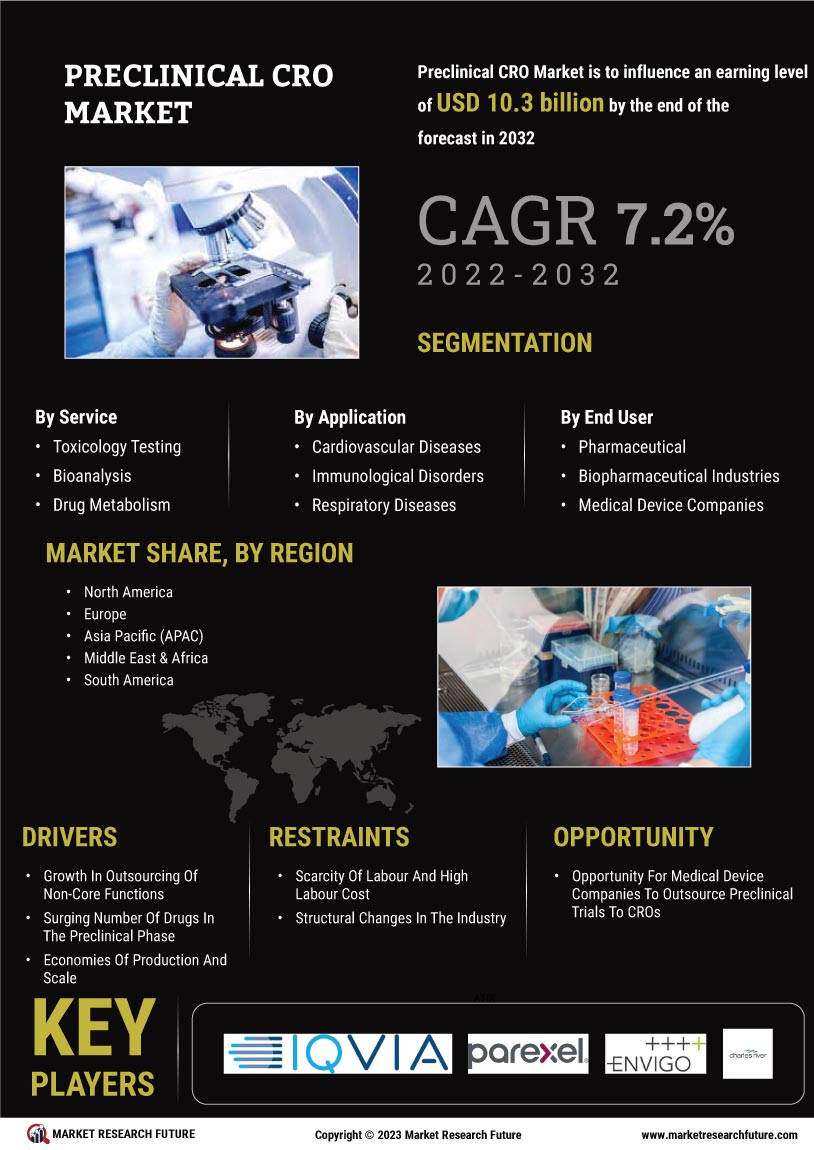
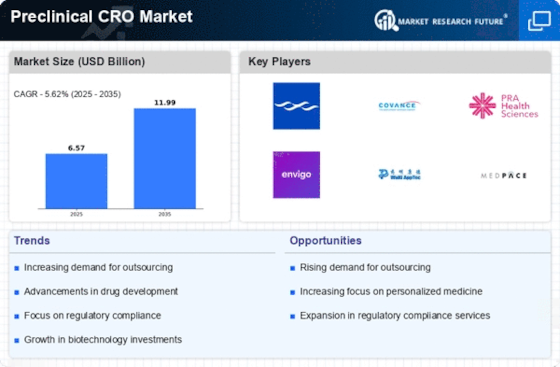
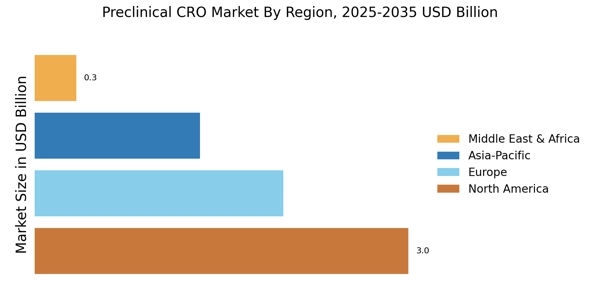
The Preclinical CRO Market is experiencing a notable surge in demand for drug development services. This trend is largely driven by the increasing number of pharmaceutical companies seeking to expedite their research and development processes. In recent years, the industry has seen a significant uptick in the number of new drug candidates entering the pipeline, with estimates suggesting that The Preclinical CRO Market could reach over 1.5 trillion USD by 2025. As a result, preclinical contract research organizations (CROs) are becoming essential partners for these companies, providing critical services such as toxicology studies, pharmacokinetics, and efficacy testing. This growing reliance on preclinical CROs indicates a shift towards outsourcing, allowing pharmaceutical firms to focus on core competencies while leveraging specialized expertise in the preclinical phase.
Growing Focus on Personalized Medicine
The Preclinical CRO Market is also being shaped by the growing focus on personalized medicine. As the healthcare landscape shifts towards more tailored therapeutic approaches, the demand for preclinical studies that support personalized treatment strategies is increasing. This trend necessitates the development of preclinical models that accurately reflect patient variability, which can be complex and resource-intensive. Preclinical CROs are responding by enhancing their capabilities in biomarker discovery and patient-derived xenograft models, which are essential for evaluating the efficacy of personalized therapies. The market for personalized medicine is projected to reach over 2 trillion USD by 2025, indicating a substantial opportunity for preclinical CROs to align their services with this burgeoning field. As the industry continues to evolve, those preclinical CROs that can effectively support personalized medicine initiatives are likely to see significant growth.
Increased Investment in Research and Development
The Preclinical CRO Market is witnessing a substantial increase in investment directed towards research and development (R&D) activities. Pharmaceutical and biotechnology companies are allocating larger portions of their budgets to R&D, with global spending expected to surpass 200 billion USD annually by 2025. This heightened investment is primarily aimed at discovering novel therapeutics and improving existing treatment modalities. As companies strive to bring innovative products to market, the reliance on preclinical CROs for efficient and reliable testing becomes more pronounced. These organizations provide essential support in navigating the complexities of preclinical trials, thereby enabling sponsors to optimize their R&D expenditures. The trend of increased R&D investment is likely to bolster the growth of the preclinical CRO market, as more companies seek to outsource their preclinical activities to specialized providers.