Regulatory Framework Enhancements
Japan's regulatory environment for drug development is evolving, which appears to positively impact the preclinical contract research organization market. Recent reforms aim to streamline the approval process for new drugs, thereby reducing the time and cost associated with bringing products to market. The Pharmaceuticals and Medical Devices Agency (PMDA) has introduced initiatives to facilitate faster reviews and approvals, which may encourage more companies to engage with preclinical CROs. As a result, the preclinical cro market is likely to benefit from increased project volumes, as firms seek to navigate the enhanced regulatory landscape efficiently. This proactive approach by regulatory bodies suggests a commitment to fostering innovation and could lead to a more dynamic preclinical CRO ecosystem in Japan.
Expansion of Pharmaceutical Companies
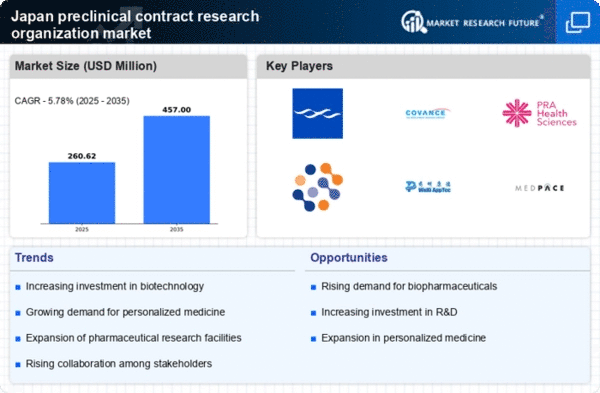
The expansion strategies of pharmaceutical companies in Japan are contributing to the growth of the preclinical contract research organization market. As these companies seek to enhance their research and development capabilities, they are increasingly outsourcing preclinical activities to specialized CROs. In 2025, it is estimated that around 40% of preclinical research will be conducted by CROs, reflecting a shift in operational strategies. This trend is likely to be fueled by the need for cost efficiency and access to advanced technologies that CROs provide. The collaboration between pharmaceutical firms and preclinical CROs is expected to foster innovation and accelerate the drug development process, thereby enhancing the overall landscape of the preclinical cro market.
Increased Investment in Biotechnology
The preclinical contract research organization market in Japan is experiencing a surge in investment, particularly in biotechnology. This trend is driven by the growing recognition of biotechnology's potential to address unmet medical needs. In 2025, Japan's biotechnology sector is projected to reach a valuation of approximately $50 billion, indicating a robust growth trajectory. As companies seek to capitalize on this potential, they are increasingly turning to preclinical contract research organizations (CROs) for expertise in drug development. This influx of investment not only enhances the capabilities of preclinical CROs but also fosters innovation, thereby propelling the overall market forward. The collaboration between biotechnology firms and preclinical CROs is likely to yield novel therapies, further stimulating demand in the preclinical cro market.
Growing Focus on Personalized Medicine
The shift towards personalized medicine is becoming increasingly pronounced in Japan, influencing the preclinical contract research organization market. As healthcare providers and pharmaceutical companies recognize the importance of tailoring treatments to individual patient profiles, the demand for specialized preclinical services is likely to rise. In 2025, the personalized medicine market in Japan is expected to grow at a CAGR of around 15%, reflecting a significant opportunity for preclinical CROs to offer tailored solutions. This trend necessitates advanced preclinical testing methodologies, which may drive collaborations between pharmaceutical companies and CROs. Consequently, the preclinical cro market could see a diversification of services offered, catering to the unique needs of personalized medicine initiatives.
Rising Demand for Innovative Therapies
The preclinical contract research organization market in Japan is witnessing a rising demand for innovative therapies, particularly in areas such as oncology and rare diseases. As the healthcare landscape evolves, there is an increasing emphasis on developing novel treatment options that address complex medical conditions. In 2025, the market for innovative therapies is projected to grow by approximately 20%, indicating a robust interest from both pharmaceutical companies and investors. This demand is likely to drive collaboration with preclinical CROs, which play a crucial role in the early stages of drug development. By leveraging the expertise of CROs, companies can expedite the research process and bring innovative therapies to market more efficiently, thereby enhancing the overall dynamics of the preclinical cro market.