Focus on Cost Efficiency
Cost efficiency remains a critical driver in the preclinical cro market, as organizations seek to optimize their research budgets. By outsourcing preclinical services, companies can significantly reduce operational costs associated with in-house research. The preclinical cro market is witnessing a shift towards more flexible pricing models, allowing clients to pay only for the services they require. This approach not only enhances financial predictability but also enables smaller biotech firms to access high-quality research services without the burden of extensive capital investment. As a result, the preclinical cro market is likely to see an influx of new clients, further stimulating growth in this sector.
Rising Demand for Drug Development
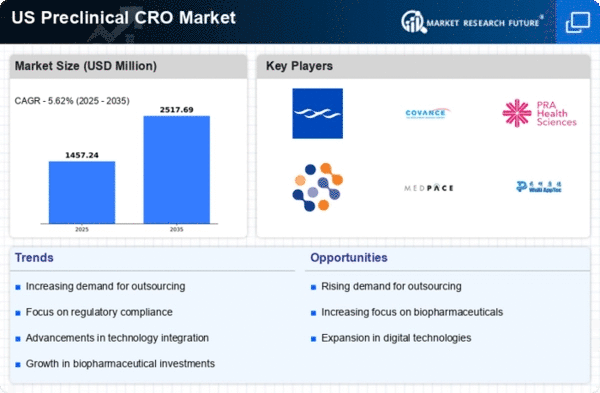
The preclinical cro market is experiencing a notable surge in demand driven by the increasing need for drug development services. As pharmaceutical companies strive to bring innovative therapies to market, the reliance on preclinical contract research organizations (CROs) has intensified. In 2025, the market is projected to reach approximately $5 billion, reflecting a growth rate of around 10% annually. This growth is largely attributed to the expanding pipeline of drug candidates, particularly in oncology and rare diseases, which necessitates robust preclinical testing. The preclinical cro market is thus positioned to benefit from this trend, as companies seek to streamline their research processes and reduce time-to-market for new drugs.
Advancements in Personalized Medicine
The rise of personalized medicine is significantly impacting the preclinical cro market, as it necessitates tailored approaches to drug development. CROs are increasingly required to conduct preclinical studies that account for genetic variations among patient populations. This trend is particularly evident in oncology, where targeted therapies are becoming the norm. The preclinical cro market is adapting to these demands by investing in advanced technologies such as genomics and bioinformatics. As a result, the market is projected to grow at a compound annual growth rate (CAGR) of 12% over the next five years, driven by the need for customized preclinical testing services.
Regulatory Landscape and Compliance Needs
The evolving regulatory landscape in the pharmaceutical industry is a significant driver for the preclinical cro market. As regulatory agencies impose stricter guidelines for drug approval, the demand for compliance-focused preclinical services has increased. CROs play a crucial role in ensuring that preclinical studies meet these regulatory requirements, thereby facilitating smoother transitions to clinical trials. The preclinical cro market is thus positioned to thrive as companies seek to mitigate risks associated with regulatory non-compliance. This trend is expected to contribute to a market growth rate of approximately 8% annually, as organizations prioritize adherence to regulatory standards in their research endeavors.
Increased Collaboration Between Academia and Industry
The preclinical cro market is benefiting from a growing trend of collaboration between academic institutions and industry players. This partnership fosters innovation and accelerates the translation of research findings into viable therapeutic options. Academic researchers often lack the resources to conduct extensive preclinical studies, making CROs essential partners in this process. The preclinical cro market is thus witnessing an increase in joint ventures and collaborative projects, which not only enhance research capabilities but also expand the client base for CROs. This collaborative approach is expected to drive market growth, as it facilitates access to cutting-edge technologies and expertise.