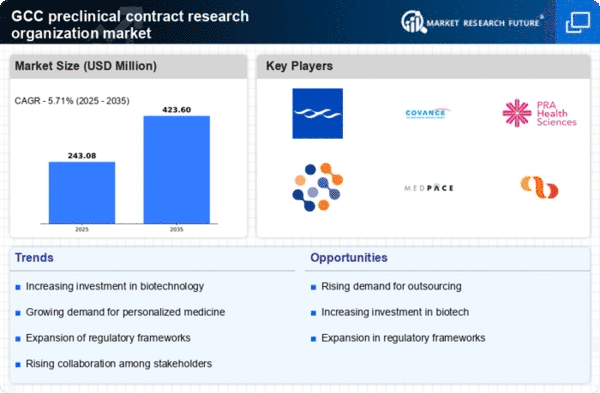
Investment in Biotechnology
Investment in biotechnology is significantly influencing the preclinical contract research organization market. The GCC region has seen a marked increase in funding for biotech startups and established companies, with investments reaching around $1.2 billion in 2025 alone. This influx of capital is likely to enhance research capabilities and foster collaborations with preclinical CROs. As biotechnology continues to evolve, the need for specialized preclinical services, including in vitro and in vivo testing, becomes more pronounced. Consequently, the preclinical cro market is poised to benefit from this investment trend, as companies seek to leverage CRO expertise to bring innovative products to market more efficiently.
Focus on Personalized Medicine
The shift towards personalized medicine is reshaping the landscape of the preclinical contract research organization market. As healthcare providers increasingly recognize the importance of tailored therapies, there is a growing need for preclinical studies that can support the development of personalized treatment options. In the GCC, the market for personalized medicine is expected to expand significantly, with estimates suggesting a growth rate of 10% annually. This trend necessitates the involvement of preclinical CROs that can provide specialized services, such as biomarker identification and patient stratification studies. The ability to deliver precise and effective therapies is likely to drive demand for preclinical services, thereby enhancing the market's growth prospects.
Rising Demand for Drug Development
The preclinical contract research organization market is experiencing a notable surge in demand for drug development services. This trend is largely driven by the increasing number of pharmaceutical companies seeking to expedite their research and development processes. In the GCC region, the pharmaceutical sector is projected to grow at a CAGR of approximately 7.5% from 2025 to 2030. As a result, preclinical contract research organizations (CROs) are becoming essential partners in the drug development lifecycle, providing critical services such as toxicology studies and pharmacokinetics. The growing emphasis on innovative therapies, particularly in oncology and rare diseases, further fuels this demand, indicating a robust future for the preclinical cro market.
Regulatory Evolution and Compliance
The evolving regulatory landscape in the GCC is a critical driver for the preclinical contract research organization market. Regulatory bodies are increasingly emphasizing compliance and safety in drug development, which necessitates rigorous preclinical testing. As regulations become more stringent, pharmaceutical companies are turning to preclinical CROs to ensure adherence to these guidelines. The preclinical cro market is likely to see growth as organizations seek expertise in navigating complex regulatory requirements. Furthermore, the introduction of new guidelines aimed at accelerating drug approval processes may also create opportunities for CROs to expand their service offerings, thereby enhancing their role in the preclinical phase of drug development.
Collaboration with Academic Institutions
Collaboration between preclinical CROs and academic institutions is emerging as a vital driver for the preclinical contract research organization market. Such partnerships facilitate the exchange of knowledge and resources, enabling more innovative research outcomes. In the GCC, several universities are establishing research centers focused on drug discovery and development, which may lead to increased demand for preclinical services. These collaborations often result in joint ventures that leverage academic expertise alongside CRO capabilities, potentially enhancing the quality and efficiency of preclinical studies. As the landscape of drug development becomes more collaborative, the preclinical cro market is likely to benefit from these synergies, fostering a more dynamic research environment.