Stringent Regulatory Frameworks
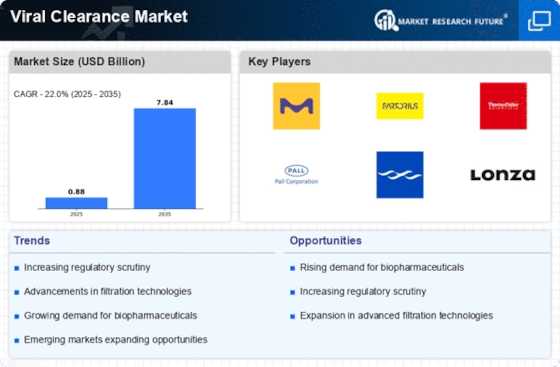
The presence of stringent regulatory frameworks significantly influences the Viral Clearance Market. Regulatory bodies, such as the FDA and EMA, impose rigorous guidelines to ensure the safety of biopharmaceutical products. Compliance with these regulations necessitates comprehensive viral clearance testing, which is essential for product approval. In 2025, the market for viral clearance services is expected to grow at a compound annual growth rate of around 12%, driven by the need for compliance with evolving regulatory standards. This trend underscores the importance of maintaining high safety standards in the Viral Clearance Market, as companies strive to meet regulatory requirements while ensuring the integrity of their products.
Rising Awareness of Product Safety
The increasing awareness of product safety among consumers and healthcare professionals is a significant driver in the Viral Clearance Market. As patients and practitioners become more informed about the risks associated with viral contamination, there is a heightened demand for products that have undergone rigorous viral clearance processes. This trend is particularly evident in the biopharmaceutical sector, where safety is paramount. In 2025, the emphasis on product safety is expected to drive a notable increase in the adoption of viral clearance services, with market growth projected at approximately 10%. This growing awareness underscores the critical role of viral clearance in ensuring the safety and efficacy of biopharmaceutical products within the Viral Clearance Market.
Increasing Demand for Biopharmaceuticals
The rising demand for biopharmaceuticals is a pivotal driver in the Viral Clearance Market. As the biopharmaceutical sector expands, the need for effective viral clearance methods becomes paramount to ensure product safety and efficacy. In 2025, the biopharmaceutical market is projected to reach approximately 500 billion USD, indicating a robust growth trajectory. This surge necessitates advanced viral clearance techniques to mitigate contamination risks, thereby propelling the demand for specialized services and technologies within the Viral Clearance Market. Companies are increasingly investing in innovative solutions to enhance their viral clearance processes, which is likely to further stimulate market growth.
Technological Innovations in Viral Clearance
Technological innovations play a crucial role in shaping the Viral Clearance Market. Advancements in filtration, chromatography, and inactivation technologies are enhancing the efficiency and effectiveness of viral clearance processes. For instance, the introduction of novel filtration membranes and improved chromatography techniques has the potential to increase the throughput and reliability of viral clearance methods. As of 2025, the market for viral clearance technologies is anticipated to witness substantial growth, with an estimated value of over 1 billion USD. This growth is indicative of the industry's commitment to adopting cutting-edge technologies to address the challenges associated with viral contamination, thereby reinforcing the importance of innovation in the Viral Clearance Market.
Expansion of Contract Manufacturing Organizations (CMOs)
The expansion of Contract Manufacturing Organizations (CMOs) is a notable driver in the Viral Clearance Market. As pharmaceutical companies increasingly outsource their manufacturing processes to CMOs, the demand for viral clearance services is likely to rise. CMOs are often required to implement stringent viral clearance protocols to meet client specifications and regulatory standards. In 2025, the CMO market is projected to grow significantly, with estimates suggesting a value exceeding 100 billion USD. This growth presents a substantial opportunity for viral clearance service providers, as CMOs seek to enhance their capabilities and ensure compliance with safety regulations. Consequently, the expansion of CMOs is expected to bolster the demand for viral clearance solutions within the Viral Clearance Market.