Stringent Regulatory Frameworks
The regulatory landscape in Germany plays a pivotal role in shaping the viral clearance market. Stringent regulations imposed by authorities, such as the European Medicines Agency (EMA), necessitate rigorous viral clearance processes for biopharmaceutical products. Compliance with these regulations is essential for market access and product approval, thereby driving demand for viral clearance services. In 2025, it is anticipated that the regulatory requirements will become even more stringent, leading to an estimated 10% increase in the demand for viral clearance solutions. This trend underscores the importance of maintaining high safety standards and adhering to regulatory guidelines, which are critical for ensuring the integrity of therapeutic products. As a result, the viral clearance market is expected to expand as companies invest in compliance strategies and technologies to meet these evolving regulatory demands.
Increased Focus on Patient Safety
Patient safety remains a critical concern in the healthcare sector, driving the viral clearance market in Germany. With rising awareness regarding the risks associated with viral contamination in therapeutic products, stakeholders are prioritizing stringent viral clearance measures. The viral clearance market is expected to benefit from this heightened focus, as healthcare providers and manufacturers strive to ensure the safety of their products. In 2025, it is estimated that the demand for viral clearance services will increase by approximately 15%, reflecting the industry's commitment to safeguarding patient health. This trend indicates a shift towards more rigorous testing and validation processes, which are essential for maintaining high safety standards. As a result, the viral clearance market is likely to expand, with companies investing in innovative solutions to enhance their viral clearance protocols.
Growing Investment in Biotechnology
The biotechnology sector in Germany is witnessing substantial investment, which is a significant driver for the viral clearance market. As venture capital and public funding increase, biotechnology companies are expanding their research and development efforts, necessitating effective viral clearance strategies. In 2025, investments in biotechnology are projected to exceed €5 billion, reflecting a growing recognition of the sector's potential. This influx of capital is likely to lead to the development of innovative therapies and products, which in turn will require robust viral clearance processes to ensure safety and compliance. Consequently, the viral clearance market is poised for growth as biotechnology firms prioritize viral clearance in their product development pipelines. This trend indicates a broader commitment to maintaining high safety standards in the rapidly evolving biotechnology landscape.
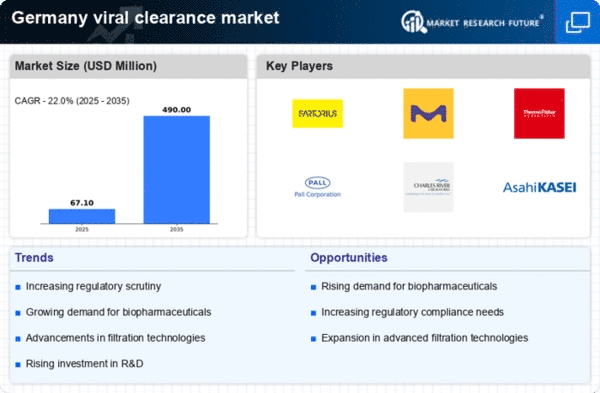
Rising Demand for Biopharmaceuticals
The increasing demand for biopharmaceuticals in Germany is a key driver for the viral clearance market. As the biopharmaceutical sector expands, the need for effective viral clearance processes becomes paramount to ensure product safety and efficacy. In 2025, the biopharmaceutical market in Germany is projected to reach approximately €20 billion, reflecting a growth rate of around 8% annually. This surge necessitates robust viral clearance strategies to mitigate contamination risks, thereby propelling the viral clearance market forward. Furthermore, the emphasis on high-quality standards in biopharmaceutical production underscores the importance of viral clearance, as manufacturers seek to comply with stringent regulations. Consequently, the viral clearance market is likely to experience significant growth as companies invest in advanced technologies and methodologies to enhance their viral clearance capabilities.
Advancements in Analytical Techniques
Technological innovations in analytical techniques are significantly influencing the viral clearance market in Germany. The development of advanced methodologies, such as next-generation sequencing and high-throughput screening, enhances the ability to detect and quantify viral contaminants effectively. These advancements are crucial for ensuring compliance with regulatory standards and improving the overall efficiency of viral clearance processes. In 2025, the market for analytical technologies in the viral clearance sector is projected to grow by approximately 12%, driven by the increasing need for precise and reliable testing methods. This growth indicates a shift towards more sophisticated approaches in viral clearance, which could lead to improved product safety and efficacy. Consequently, the viral clearance market is likely to see increased investment in research and development to further advance these analytical techniques.