Rising Demand for Biologics
The increasing demand for biologics in Japan is a pivotal driver for the viral clearance market. As the biopharmaceutical sector expands, the need for effective viral clearance methods becomes paramount. In 2025, the market for biologics is projected to reach approximately $10 billion, indicating a robust growth trajectory. This surge necessitates stringent viral clearance processes to ensure product safety and efficacy. The viral clearance market is thus positioned to benefit from this trend, as manufacturers seek reliable solutions to mitigate viral contamination risks. Furthermore, the growing prevalence of chronic diseases in Japan amplifies the need for innovative biologic therapies, further driving the demand for advanced viral clearance technologies. As a result, companies are likely to invest in research and development to enhance their viral clearance capabilities, ensuring compliance with regulatory standards and meeting market expectations.
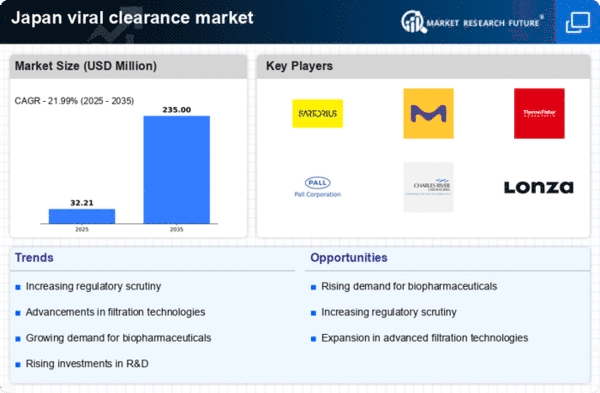
Increased Regulatory Scrutiny
In Japan, the viral clearance market is significantly influenced by heightened regulatory scrutiny surrounding biopharmaceutical products. Regulatory bodies are enforcing stricter guidelines to ensure the safety and efficacy of biologics, which directly impacts the viral clearance market. As of 2025, compliance with these regulations is not merely a recommendation but a necessity for market players. Companies must implement robust viral clearance strategies to meet the evolving standards set forth by authorities. This regulatory environment fosters innovation within the viral clearance market, as firms strive to develop more effective and efficient clearance methods. The financial implications are notable, with companies potentially facing penalties for non-compliance, which could reach millions of yen. Therefore, the need for adherence to regulatory frameworks is a critical driver, compelling organizations to prioritize viral clearance in their operational strategies.
Growing Awareness of Viral Safety
The increasing awareness of viral safety among consumers and healthcare professionals is a significant driver for the viral clearance market in Japan. As public health concerns rise, there is a heightened focus on ensuring that biopharmaceutical products are free from viral contaminants. This trend is reflected in the growing demand for viral clearance solutions, as stakeholders prioritize safety in their purchasing decisions. The viral clearance market is responding to this shift by developing more comprehensive testing and clearance protocols. In 2025, it is anticipated that the market will expand by approximately 7%, driven by this heightened awareness. Companies that effectively communicate their commitment to viral safety are likely to gain a competitive edge, as consumers increasingly seek transparency regarding product safety. Thus, the emphasis on viral safety is reshaping the dynamics of the viral clearance market, influencing both production practices and consumer behavior.
Technological Innovations in Clearance Methods
Technological advancements are reshaping the landscape of the viral clearance market in Japan. Innovations such as improved filtration techniques and advanced chromatography methods are enhancing the efficiency of viral clearance processes. In 2025, the market is expected to witness a growth rate of approximately 8% annually, driven by these technological breakthroughs. The viral clearance market is adapting to these changes, as companies invest in state-of-the-art technologies to optimize their production processes. Enhanced viral clearance methods not only improve safety but also reduce production costs, making them attractive to manufacturers. As competition intensifies, the adoption of cutting-edge technologies becomes essential for maintaining market share. Consequently, the integration of innovative solutions is likely to propel the growth of the viral clearance market, positioning it as a vital component of the biopharmaceutical manufacturing process.
Expansion of Contract Manufacturing Organizations
The proliferation of contract manufacturing organizations (CMOs) in Japan is a notable driver of the viral clearance market. As biopharmaceutical companies increasingly outsource their production processes, the demand for reliable viral clearance solutions escalates. CMOs are under pressure to ensure that their manufacturing practices meet stringent safety standards, which directly impacts the viral clearance market. In 2025, the CMO sector is projected to grow by approximately 10%, creating a substantial opportunity for viral clearance service providers. This trend indicates a shift towards specialized services, as CMOs seek to enhance their capabilities in viral clearance to attract more clients. Consequently, the viral clearance market is likely to experience growth as these organizations invest in advanced clearance technologies and processes. The collaboration between CMOs and viral clearance experts is expected to foster innovation and improve overall product safety in the biopharmaceutical sector.