Increased Regulatory Scrutiny
The evolving regulatory landscape in Italy significantly impacts the viral clearance market. Regulatory bodies are intensifying their scrutiny of biopharmaceutical products, necessitating rigorous viral clearance testing to ensure compliance with safety standards. In recent years, the Italian Medicines Agency (AIFA) has implemented stricter guidelines for viral clearance validation, which has led to an increased demand for specialized services and technologies in the market. As a result, companies are compelled to invest in advanced viral clearance solutions to meet these regulatory requirements. The market is expected to grow as organizations prioritize compliance, with estimates suggesting a potential increase in market size by 15% over the next five years. This heightened focus on regulatory compliance not only drives demand for viral clearance services but also fosters innovation within the industry, as companies seek to develop more efficient and effective clearance methods.
Growing Awareness of Product Safety
The heightened awareness of product safety among consumers and healthcare professionals is a significant driver for the viral clearance market. In Italy, there is an increasing emphasis on ensuring that biopharmaceutical products are free from viral contaminants, which has led to greater scrutiny of manufacturing processes. This awareness is influencing purchasing decisions, as stakeholders prioritize products that demonstrate robust viral clearance measures. Consequently, manufacturers are compelled to adopt stringent viral clearance protocols to maintain consumer trust and meet market expectations. The viral clearance market is projected to expand as companies respond to this demand for enhanced safety measures. It is estimated that the market could grow by 10% in the coming years, reflecting the critical importance of product safety in the biopharmaceutical sector.
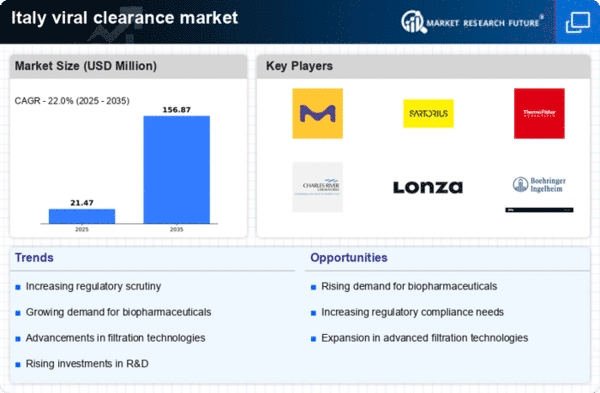
Rising Demand for Biopharmaceuticals
The increasing demand for biopharmaceuticals in Italy is a key driver for the viral clearance market. As the biopharmaceutical sector expands, the need for effective viral clearance processes becomes paramount to ensure product safety and efficacy. In 2025, the biopharmaceutical market in Italy is projected to reach approximately €10 billion, reflecting a growth rate of around 8% annually. This surge necessitates robust viral clearance strategies to mitigate contamination risks, thereby propelling the viral clearance market forward. Furthermore, the emphasis on high-quality standards in biopharmaceutical production underscores the importance of viral clearance, as manufacturers seek to comply with stringent regulations. Consequently, the viral clearance market is likely to experience significant growth as companies invest in advanced technologies and methodologies to enhance their viral clearance capabilities.
Investment in Research and Development
Investment in research and development (R&D) is a vital driver for the viral clearance market in Italy. As biopharmaceutical companies strive to innovate and improve their product offerings, R&D efforts are increasingly focused on developing more effective viral clearance technologies. In 2025, it is expected that R&D spending in the biopharmaceutical sector will exceed €1 billion, with a significant portion allocated to viral clearance solutions. This investment is likely to lead to breakthroughs in viral clearance methodologies, enhancing the overall safety and efficacy of biopharmaceutical products. Furthermore, collaboration between academic institutions and industry players is fostering a conducive environment for innovation, which may further propel the viral clearance market. The emphasis on R&D not only drives technological advancements but also positions Italy as a competitive player in the global biopharmaceutical landscape.
Technological Innovations in Viral Clearance
Technological advancements play a crucial role in shaping the viral clearance market in Italy. Innovations such as improved filtration techniques, chromatography methods, and viral inactivation technologies are enhancing the efficiency and effectiveness of viral clearance processes. In 2025, the market for viral clearance technologies is anticipated to grow by approximately 12%, driven by the adoption of these cutting-edge solutions. Companies are increasingly investing in research and development to create novel viral clearance methods that can address emerging viral threats. This trend not only improves product safety but also reduces operational costs for manufacturers. As the viral clearance market continues to evolve, the integration of advanced technologies will likely remain a pivotal factor in meeting the growing demands of the biopharmaceutical industry.