Emergence of Advanced Technologies
The emergence of advanced technologies in the field of viral clearance is reshaping the market landscape in South America. Innovations such as nanofiltration, chromatography, and viral inactivation methods are becoming increasingly prevalent, offering enhanced efficiency and effectiveness in viral clearance processes. These technologies not only improve the safety of biopharmaceutical products but also reduce operational costs. The viral clearance market is expected to witness a surge in demand for these advanced solutions, as companies strive to optimize their production processes. By 2025, the adoption of such technologies could lead to a 15% reduction in viral clearance processing times, thereby enhancing overall productivity.
Rising Demand for Biopharmaceuticals
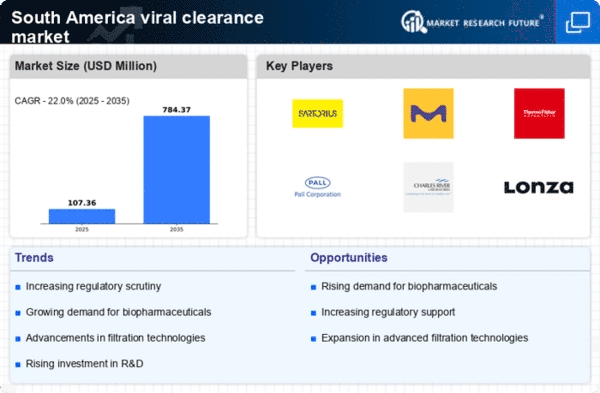
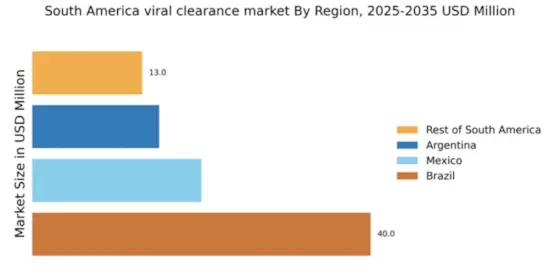
The increasing demand for biopharmaceuticals in South America is a key driver for the viral clearance market. As the biopharmaceutical sector expands, the need for effective viral clearance methods becomes paramount to ensure product safety and efficacy. In 2025, the biopharmaceutical market in South America is projected to reach approximately $20 billion, reflecting a compound annual growth rate (CAGR) of around 8%. This growth necessitates robust viral clearance processes to mitigate contamination risks, thereby propelling the demand for advanced viral clearance technologies and services. The viral clearance market must adapt to these evolving needs, focusing on innovative solutions that align with the biopharmaceuticals' stringent safety standards.
Growing Awareness of Safety Standards
The growing awareness of safety standards among consumers and regulatory bodies in South America is a significant driver for the viral clearance market. As public health concerns rise, there is an increasing emphasis on ensuring that biopharmaceutical products are free from viral contaminants. This heightened awareness is leading to stricter regulations and guidelines, compelling manufacturers to invest in effective viral clearance methods. In 2025, it is estimated that compliance costs related to safety standards could account for up to 10% of total production expenses in the biopharmaceutical sector. Thus, the viral clearance market is likely to experience growth as companies prioritize safety and compliance in their operations.
Regulatory Changes and Compliance Requirements
Regulatory changes and evolving compliance requirements in South America are driving the viral clearance market. As regulatory agencies update their guidelines to address emerging health threats, manufacturers must adapt their viral clearance strategies accordingly. This dynamic environment necessitates continuous investment in research and development to meet compliance standards. In 2025, it is anticipated that regulatory compliance costs will represent approximately 12% of total operational expenses for biopharmaceutical companies. Consequently, the viral clearance market is likely to expand as companies seek to implement effective viral clearance solutions that align with the latest regulatory frameworks.
Increased Investment in Healthcare Infrastructure
Investment in healthcare infrastructure across South America is significantly influencing the viral clearance market. Governments and private entities are channeling funds into healthcare systems to enhance their capabilities, particularly in the biopharmaceutical sector. This investment is expected to exceed $15 billion by 2026, fostering the establishment of state-of-the-art laboratories and manufacturing facilities. As these infrastructures develop, the demand for effective viral clearance solutions will likely rise, as companies seek to comply with safety regulations and improve product quality. Consequently, the viral clearance market stands to benefit from this influx of capital, driving innovation and the adoption of advanced technologies.