Increased Investment in R&D
Investment in research and development (R&D) within the biopharmaceutical sector is a significant driver for the viral clearance market. South Korea has been actively promoting innovation through various government initiatives, leading to an increase in funding for R&D activities. In 2025, R&D expenditure in the life sciences sector is expected to exceed $5 billion, reflecting a commitment to advancing healthcare technologies. This influx of capital is likely to foster the development of novel viral clearance methods and technologies, enhancing the overall efficiency and effectiveness of the viral clearance market. As companies strive to develop cutting-edge therapies, the demand for robust viral clearance processes will continue to rise, ensuring that products meet the highest safety standards.
Stringent Regulatory Frameworks
The presence of stringent regulatory frameworks in South Korea significantly influences the viral clearance market. Regulatory bodies, such as the Ministry of Food and Drug Safety (MFDS), enforce rigorous guidelines to ensure the safety and efficacy of biopharmaceutical products. Compliance with these regulations is crucial for manufacturers, as failure to adhere can result in severe penalties and product recalls. In 2025, the MFDS is expected to implement even more stringent guidelines, further emphasizing the importance of effective viral clearance processes. This regulatory landscape drives the viral clearance market to innovate and adopt advanced technologies that can meet these evolving standards, thereby ensuring product safety and maintaining public trust.
Emergence of Advanced Technologies
The emergence of advanced technologies in the field of viral clearance is a notable driver for the market. Innovations such as nanofiltration, chromatography, and viral inactivation methods are revolutionizing the way manufacturers approach viral clearance. In South Korea, the adoption of these technologies is expected to increase significantly, with the market projected to grow at a CAGR of 8% from 2025 to 2030. This growth is indicative of the viral clearance market's responsiveness to technological advancements that enhance efficiency and effectiveness. As manufacturers seek to optimize their production processes and ensure compliance with safety regulations, the integration of these advanced technologies will likely become a standard practice, further driving market growth.
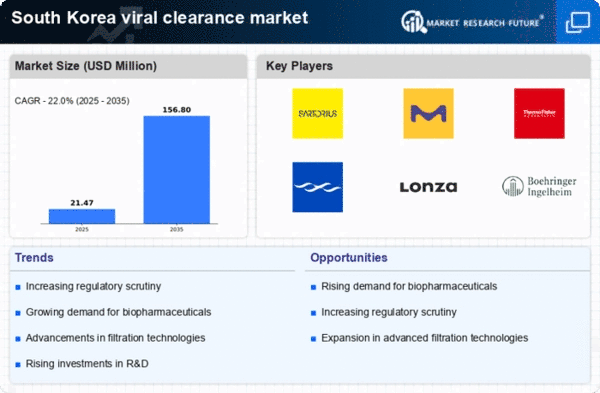
Rising Demand for Biopharmaceuticals
The increasing demand for biopharmaceuticals in South Korea is a key driver for the viral clearance market. As the biopharmaceutical sector expands, the need for effective viral clearance processes becomes paramount to ensure product safety and efficacy. In 2025, the biopharmaceutical market in South Korea is projected to reach approximately $10 billion, indicating a robust growth trajectory. This surge necessitates advanced viral clearance technologies to mitigate contamination risks. The viral clearance market is thus positioned to benefit from this trend, as manufacturers seek to comply with stringent safety regulations and maintain consumer trust. Furthermore, the growing prevalence of chronic diseases and the aging population in South Korea are likely to further fuel the demand for biopharmaceuticals, thereby enhancing the need for reliable viral clearance solutions.
Growing Awareness of Safety Standards
There is a growing awareness of safety standards among consumers and healthcare professionals in South Korea, which is driving the viral clearance market. As patients become more informed about the risks associated with biopharmaceuticals, they increasingly demand transparency regarding the safety measures implemented during production. This heightened awareness compels manufacturers to invest in robust viral clearance processes to ensure their products are free from viral contaminants. The viral clearance market is thus likely to see increased demand for advanced technologies that can provide reliable assurance of product safety. In 2025, consumer expectations regarding safety are anticipated to rise, further propelling the need for effective viral clearance solutions.