Stringent Regulatory Frameworks
The regulatory landscape in France plays a pivotal role in shaping the viral clearance market. Stringent regulations imposed by health authorities necessitate rigorous viral clearance testing for biopharmaceutical products. Compliance with these regulations is not only mandatory but also critical for ensuring product safety and efficacy. The French National Agency for the Safety of Medicines and Health Products (ANSM) enforces these standards, which compel manufacturers to adopt comprehensive viral clearance strategies. As a result, the market is experiencing a surge in demand for reliable viral clearance solutions that meet regulatory requirements. This trend is likely to continue, as regulatory bodies increasingly emphasize the importance of viral safety in therapeutic products, thereby driving growth in the viral clearance market.
Increased Focus on Patient Safety
Patient safety remains a paramount concern in the healthcare sector, significantly influencing the viral clearance market. In France, healthcare providers and manufacturers are increasingly prioritizing the implementation of stringent viral clearance measures to protect patients from potential viral contamination. This heightened focus is driven by regulatory bodies that mandate rigorous testing and validation of viral clearance processes. As a result, the market is witnessing a shift towards more comprehensive viral clearance solutions, which are essential for maintaining the integrity of therapeutic products. The commitment to patient safety is expected to drive investments in innovative viral clearance technologies, thereby enhancing the overall efficacy of the viral clearance market. This trend indicates a growing recognition of the critical role that effective viral clearance plays in safeguarding public health.
Rising Demand for Biopharmaceuticals
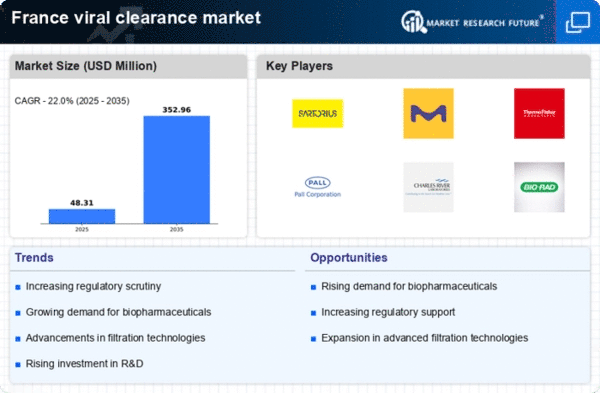
The increasing demand for biopharmaceuticals in France is a primary driver for the viral clearance market. As the biopharmaceutical sector expands, the need for effective viral clearance processes becomes critical to ensure product safety and efficacy. In 2025, the biopharmaceutical market in France is projected to reach approximately €30 billion, reflecting a growth rate of around 8% annually. This surge necessitates robust viral clearance strategies to mitigate contamination risks, thereby propelling the viral clearance market. Companies are investing in advanced technologies to enhance their viral clearance capabilities, which is likely to further stimulate market growth. The emphasis on patient safety and regulatory compliance in the biopharmaceutical industry underscores the importance of viral clearance processes, making it a vital component of production protocols.
Advancements in Research and Development
Ongoing advancements in research and development (R&D) are significantly impacting the viral clearance market. In France, increased funding for R&D initiatives is fostering innovation in viral clearance technologies. This investment is crucial for developing more efficient and effective viral clearance methods, which are essential for the production of safe biopharmaceuticals. The French government has allocated substantial resources to support biotech firms, which are at the forefront of these advancements. As a result, the viral clearance market is likely to benefit from novel approaches that enhance the reliability and speed of viral clearance processes. The integration of cutting-edge technologies, such as nanotechnology and advanced filtration systems, is expected to revolutionize the viral clearance landscape, thereby driving market growth in the coming years.
Growing Awareness of Viral Contamination Risks
There is a growing awareness of the risks associated with viral contamination in therapeutic products, which is significantly influencing the viral clearance market. In France, healthcare professionals and manufacturers are becoming increasingly cognizant of the potential consequences of viral contamination, leading to a heightened demand for effective viral clearance solutions. This awareness is driven by high-profile cases of contamination that have underscored the importance of robust viral clearance processes. Consequently, the market is witnessing an uptick in investments aimed at enhancing viral clearance capabilities. Educational initiatives and industry collaborations are also playing a role in disseminating knowledge about viral risks, further propelling the viral clearance market. This trend suggests a proactive approach to ensuring product safety and efficacy in the biopharmaceutical sector.