Growing Awareness of Mycoplasma Risks
The growing awareness of the risks associated with mycoplasma contamination among researchers and manufacturers is a crucial driver for the Mycoplasma Testing Market. Educational initiatives and industry conferences are increasingly highlighting the potential impact of mycoplasma on research outcomes and product quality. This awareness is leading to a proactive approach in testing, with more organizations prioritizing mycoplasma testing as part of their quality assurance processes. As a result, the market is expected to experience a surge in demand, with projections indicating a growth rate of approximately 8% annually. This trend reflects a shift towards a more vigilant stance on contamination risks, further solidifying the role of mycoplasma testing in laboratory and manufacturing settings.
Rising Incidence of Mycoplasma Contamination
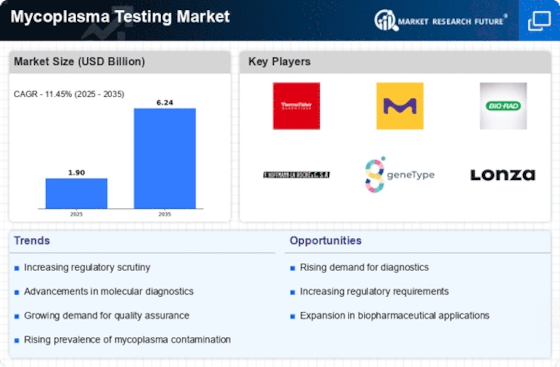
The increasing incidence of mycoplasma contamination in cell cultures and biopharmaceutical products is a primary driver for the Mycoplasma Testing Market. Mycoplasma contamination can lead to significant financial losses and compromised product quality, prompting manufacturers to invest in robust testing solutions. Reports indicate that approximately 15-35% of cell cultures may be contaminated with mycoplasma, underscoring the necessity for effective testing. As the biopharmaceutical sector expands, the demand for reliable mycoplasma testing is expected to rise, with the market projected to grow at a compound annual growth rate of around 10% over the next few years. This trend highlights the critical role of mycoplasma testing in ensuring the safety and efficacy of biological products.
Advancements in Molecular Testing Technologies
Technological advancements in molecular testing methods are significantly influencing the Mycoplasma Testing Market. Innovations such as PCR (Polymerase Chain Reaction) and next-generation sequencing have enhanced the sensitivity and specificity of mycoplasma detection. These advanced techniques allow for rapid and accurate identification of mycoplasma species, which is crucial for maintaining the integrity of biopharmaceutical products. The market for molecular testing is expected to witness substantial growth, with estimates suggesting a market size increase to over USD 1 billion by 2026. As laboratories seek to adopt more efficient testing methodologies, the demand for advanced mycoplasma testing solutions is likely to escalate, further propelling market growth.
Expansion of Biopharmaceutical Manufacturing Facilities
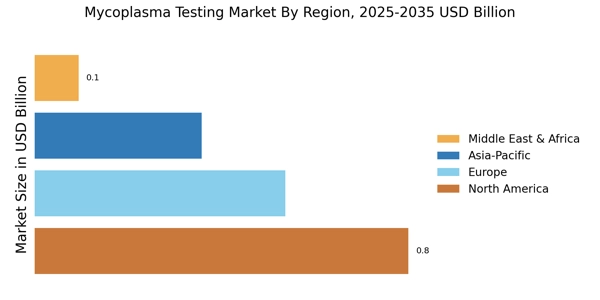
The expansion of biopharmaceutical manufacturing facilities is driving the Mycoplasma Testing Market. As new production plants are established, the need for comprehensive testing protocols becomes paramount to ensure product safety and compliance with regulatory standards. The biopharmaceutical sector is experiencing rapid growth, with investments in new facilities projected to exceed USD 10 billion in the coming years. This expansion necessitates the implementation of rigorous mycoplasma testing to prevent contamination and ensure the integrity of biological products. Consequently, the demand for mycoplasma testing solutions is likely to increase, as manufacturers seek to safeguard their operations and maintain high-quality standards in their products.
Increased Focus on Quality Control in Biopharmaceuticals
The heightened focus on quality control and assurance in the biopharmaceutical industry serves as a significant driver for the Mycoplasma Testing Market. Regulatory bodies are imposing stringent guidelines to ensure that biopharmaceutical products are free from contaminants, including mycoplasma. This regulatory landscape compels manufacturers to implement comprehensive testing protocols, thereby increasing the demand for mycoplasma testing solutions. The market is projected to reach USD 500 million by 2025, driven by the need for compliance with these regulations. As companies strive to meet quality standards, the reliance on effective mycoplasma testing will likely intensify, reinforcing its importance in the biopharmaceutical manufacturing process.