Increasing Regulatory Scrutiny
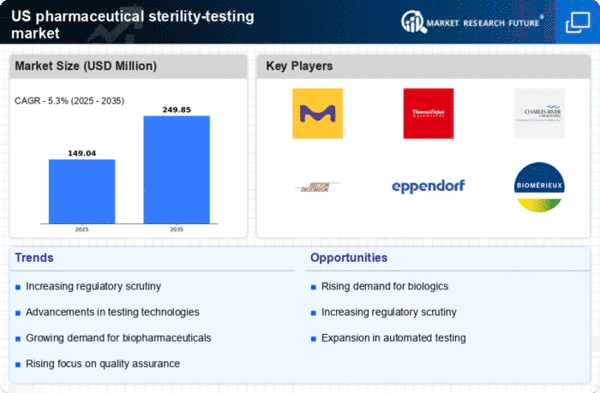
The pharmaceutical sterility-testing market is experiencing heightened regulatory scrutiny as agencies such as the FDA enforce stricter compliance measures. This trend is driven by the need to ensure product safety and efficacy, particularly in sterile drug manufacturing. The FDA's guidelines mandate rigorous sterility testing protocols, which has led to an increase in demand for advanced testing solutions. As a result, companies are investing in state-of-the-art sterility testing technologies to meet these regulatory requirements. The market is projected to grow at a CAGR of approximately 8% over the next few years, reflecting the increasing importance of compliance in the pharmaceutical sector.
Expansion of Biopharmaceuticals
The expansion of the biopharmaceutical sector is a significant driver of the pharmaceutical sterility-testing market. As the development of biologics and biosimilars accelerates, the need for stringent sterility testing becomes increasingly critical. Biopharmaceuticals often require specialized testing methods to ensure their safety and effectiveness, which in turn drives demand for advanced sterility testing solutions. The market for biopharmaceuticals is projected to reach $500 billion by 2026, indicating a robust growth trajectory that will likely bolster the sterility-testing market as well. This interdependence suggests that the growth of biopharmaceuticals will continue to shape the landscape of sterility testing.
Rising Consumer Health Awareness
Rising consumer health awareness is influencing the pharmaceutical sterility-testing market. As patients become more informed about the safety and quality of medications, there is an increasing demand for transparency in pharmaceutical manufacturing processes. This trend compels companies to adopt rigorous sterility testing protocols to ensure product safety. The market is responding to this shift by enhancing testing methodologies and increasing the frequency of sterility tests. With an anticipated growth rate of 10% in consumer demand for high-quality pharmaceutical products, the sterility-testing market is likely to see a corresponding increase in the adoption of advanced testing solutions.
Growing Focus on Quality Assurance
Quality assurance remains a pivotal concern within the pharmaceutical sterility-testing market. As companies strive to uphold high standards in product quality, the demand for reliable sterility testing solutions is on the rise. This focus on quality assurance is further fueled by consumer awareness and the need for transparency in pharmaceutical products. The market is witnessing a shift towards more comprehensive testing protocols that ensure the safety and efficacy of sterile products. With an estimated 15% increase in investments towards quality assurance measures, the pharmaceutical industry is likely to continue prioritizing sterility testing as a fundamental aspect of its operations.
Technological Innovations in Testing Methods
Technological advancements are significantly influencing the pharmaceutical sterility-testing market. Innovations such as rapid sterility testing methods and automated systems are enhancing the efficiency and accuracy of sterility testing processes. These advancements not only reduce testing times but also minimize human error, which is critical in maintaining product integrity. The integration of advanced technologies is expected to drive market growth, with estimates suggesting a potential increase in market value to over $1 billion by 2027. As pharmaceutical companies seek to streamline their operations, the adoption of these innovative testing methods is likely to become a key driver in the industry.