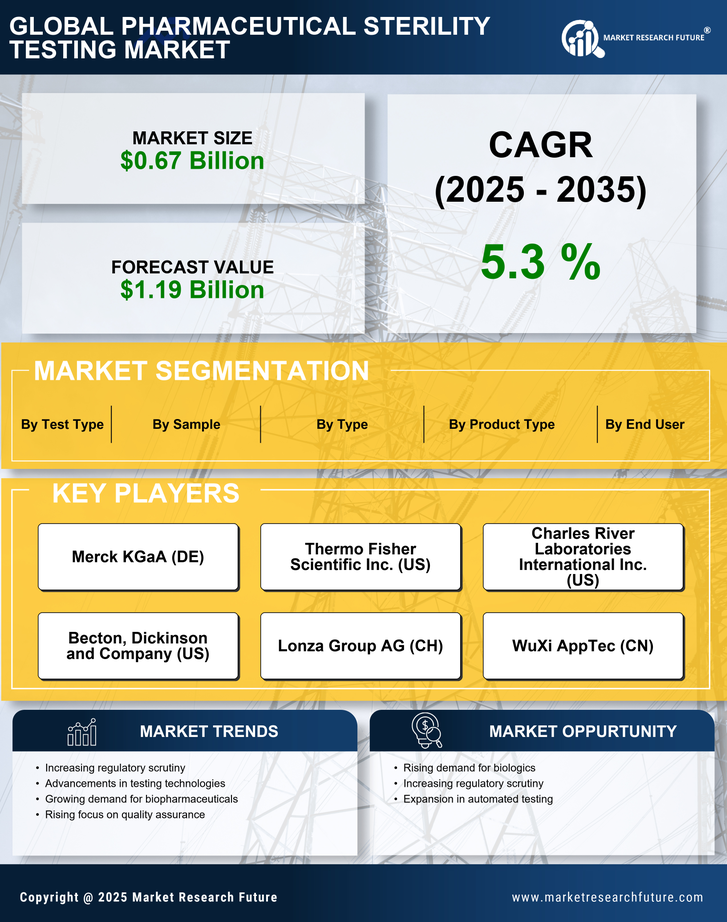
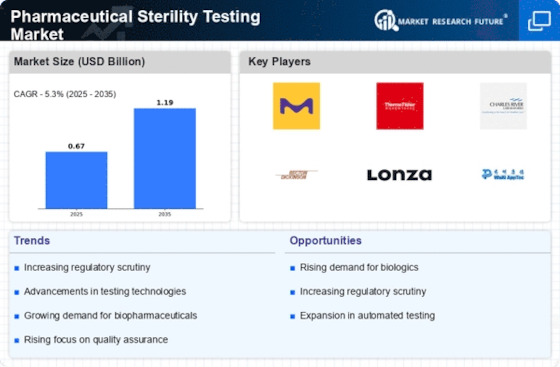
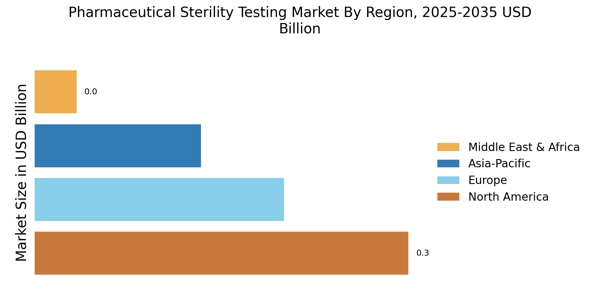
Research Methodology on Pharmaceutical Sterility Testing Market
For conducting the research report on Pharmaceutical Sterility Testing Market, the market research team applies both primary and secondary research methodologies. The primary research includes in-depth interviews, surveys, and expert opinions. The secondary research methodology involves analyzing market reports and documents, company websites, and databases. The market report also provides in-depth knowledge for various industry stakeholders such as investors, regulatory organizations, vendors, and policymakers.
Primary Research:
To gather accurate information for the Pharmaceutical Sterility Testing Market report, various primary research methodologies are utilized, such as in-depth interviews, expert opinions, surveys, etc. A team of highly experienced market researchers interviewed industry experts from different parts of the world. The findings and opinions from the interviews were combined and analyzed to draw a comprehensive research report. The primary research provided a detailed analysis of various stakeholders such as producers, suppliers, regulatory bodies, and distributors in the market.
Secondary Research:
The secondary research methodology was conducted to gain an in-depth understanding of the Pharmaceutical Sterility Testing Market. The research team collected and analyzed market data from various sources such as published documents, company websites, journals, government websites, and industry magazines. The secondary research also included an in-depth analysis of the competitive landscape of the market.
Market Analysis Tools Used:
The market analysis team used advanced analytical tools such as Porter's Five Forces analysis model, Supply Chain analysis, and SWOT analysis to analyse the Pharmaceutical Sterility Testing Market. These tools helped in understanding the competitive landscape of the market and its various dynamics. The tools also aided in analysing the potential of the market, assessing its current market value, and estimating its future development.
Research Report Validation and Revision:
Once the analysis was completed, the research team conducted a series of meetings with industry experts to validate the collected data. The collected qualitative and quantitative information was validated by multiple stakeholders from the market and the results were evaluated against industry analysis techniques. After validation, the market research team revised the report as needed to ensure accuracy.
The final research report was presented to the stakeholders for their review, and any comments or observations were incorporated into the report.
Conclusion:
Using the methodologies described above, the market research team has developed a comprehensive research report on the Pharmaceutical Sterility Testing Market. The report covers the market’s key dynamics such as drivers, restraints, and opportunities. The report also provides a detailed overview of the market’s structure and a detailed analysis of the current market situation along with the market forecast for 2023 to 2030. The report also includes a detailed SWOT analysis that provides an in-depth understanding of the state of the market.