Regulatory Support for Biologics
Regulatory frameworks supporting the development and approval of biologics are playing a crucial role in the Protein A Resin Market. Governments and regulatory bodies are increasingly recognizing the importance of biologics in modern medicine, leading to streamlined approval processes and incentives for biopharmaceutical companies. This supportive environment encourages investment in research and development, which in turn drives the demand for Protein A resins used in the purification of these products. As the regulatory landscape continues to evolve favorably, the Protein A Resin Market is likely to experience sustained growth, as companies seek to capitalize on the opportunities presented by these favorable conditions.
Rising Demand for Monoclonal Antibodies
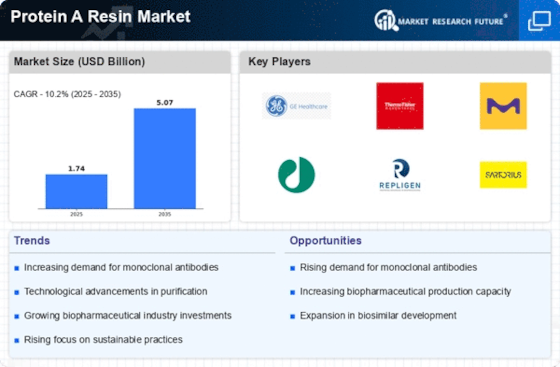
The Protein A Resin Market is experiencing a notable surge in demand for monoclonal antibodies, which are pivotal in therapeutic applications. This increase is largely driven by the growing prevalence of chronic diseases and the expanding biopharmaceutical sector. According to recent data, the monoclonal antibody market is projected to reach approximately USD 300 billion by 2025, indicating a robust growth trajectory. As these antibodies require efficient purification processes, the demand for Protein A resins, which facilitate this purification, is expected to rise correspondingly. The Protein A Resin Market is thus positioned to benefit from this trend, as manufacturers seek to enhance their production capabilities to meet the escalating needs of the healthcare sector.
Increased Focus on Quality and Compliance
The Protein A Resin Market is witnessing a heightened emphasis on quality and compliance in biopharmaceutical manufacturing. As regulatory standards become more stringent, companies are compelled to adopt high-quality purification processes to ensure product safety and efficacy. This trend is reflected in the growing adoption of Protein A resins that meet rigorous quality standards. Market data suggests that the demand for high-purity Protein A resins is on the rise, as manufacturers prioritize compliance with international regulations. This focus on quality not only enhances the reputation of biopharmaceutical companies but also drives the Protein A Resin Market towards greater innovation and product development.
Advancements in Bioprocessing Technologies
Technological innovations in bioprocessing are significantly influencing the Protein A Resin Market. Enhanced purification techniques, such as continuous chromatography and improved resin formulations, are being developed to optimize yield and reduce costs. These advancements not only streamline production but also improve the overall efficiency of biopharmaceutical manufacturing. For instance, the introduction of high-capacity Protein A resins has been shown to increase productivity by up to 50%, thereby attracting more companies to invest in these technologies. As the biopharmaceutical landscape evolves, the Protein A Resin Market is likely to see increased adoption of these cutting-edge solutions, further driving market growth.
Expansion of Biopharmaceutical Manufacturing
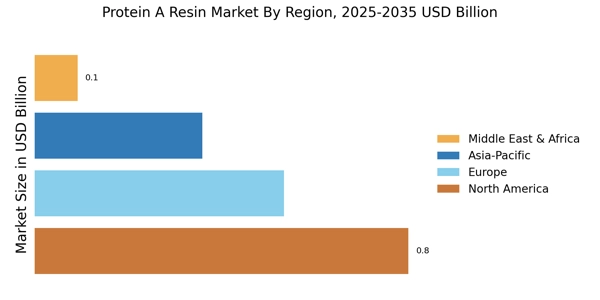
The Protein A Resin Market is benefiting from the expansion of biopharmaceutical manufacturing facilities worldwide. As more companies establish production capabilities to meet the rising demand for biologics, the need for effective purification solutions becomes paramount. Recent statistics indicate that the biopharmaceutical manufacturing market is expected to grow at a CAGR of over 8% through 2025. This growth is likely to result in increased investments in Protein A resins, as manufacturers seek to enhance their purification processes. Consequently, the Protein A Resin Market stands to gain from this trend, as it aligns with the broader movement towards more efficient and scalable biopharmaceutical production.