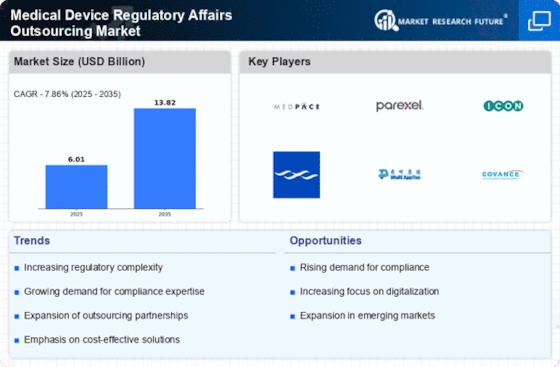
Increased Demand for Market Access
The Medical Device Regulatory Affairs Outsourcing Market is significantly influenced by the rising demand for expedited market access. As competition intensifies, medical device companies are under pressure to bring their products to market swiftly while ensuring compliance with regulatory standards. Outsourcing regulatory affairs enables these companies to streamline the approval process, thereby reducing time-to-market. This demand is particularly pronounced in regions with rapidly evolving regulatory landscapes, where local expertise can facilitate faster approvals. The ability to navigate these complexities effectively is becoming a critical factor for success, suggesting that the Medical Device Regulatory Affairs Outsourcing Market will continue to thrive as companies seek to enhance their market access strategies.
Cost Efficiency and Resource Optimization
In the Medical Device Regulatory Affairs Outsourcing Market, the pursuit of cost efficiency and resource optimization is a primary driver. Companies are increasingly recognizing that outsourcing regulatory affairs can lead to substantial savings in operational costs. By delegating these functions to specialized firms, organizations can focus their internal resources on core activities such as research and development. This shift not only enhances productivity but also allows for a more agile response to market demands. According to recent estimates, outsourcing can reduce regulatory costs by up to 30%, making it an attractive option for many medical device manufacturers. As the industry continues to evolve, the emphasis on cost-effective solutions is likely to bolster the growth of the Medical Device Regulatory Affairs Outsourcing Market.
Growing Focus on Quality Management Systems
The emphasis on robust quality management systems (QMS) is a significant driver in the Medical Device Regulatory Affairs Outsourcing Market. Regulatory authorities are increasingly mandating that medical device manufacturers implement comprehensive QMS to ensure product safety and efficacy. This trend has led to a surge in demand for outsourcing regulatory affairs, as specialized firms can provide the necessary expertise to develop and maintain these systems. By outsourcing, companies can ensure compliance with international standards such as ISO 13485, which is essential for market entry in many regions. The growing recognition of the importance of quality management is likely to propel the Medical Device Regulatory Affairs Outsourcing Market, as organizations strive to meet regulatory expectations.
Rising Complexity of Regulatory Requirements
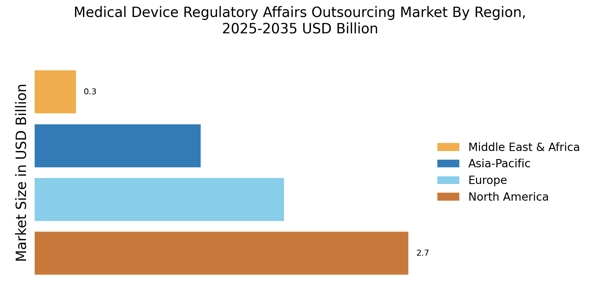
The Medical Device Regulatory Affairs Outsourcing Market is experiencing a notable increase in the complexity of regulatory requirements across various regions. As regulatory bodies implement more stringent guidelines, companies are compelled to seek specialized expertise to navigate these challenges. This complexity is driven by the need for enhanced safety and efficacy standards, which can vary significantly from one jurisdiction to another. Consequently, outsourcing regulatory affairs has become a strategic approach for many organizations, allowing them to leverage the knowledge of experienced professionals who are well-versed in local regulations. This trend is expected to continue, as the demand for compliance with evolving regulations grows, thereby propelling the Medical Device Regulatory Affairs Outsourcing Market forward.
Technological Integration in Regulatory Processes
The integration of advanced technologies into regulatory processes is reshaping the Medical Device Regulatory Affairs Outsourcing Market. Innovations such as artificial intelligence, machine learning, and data analytics are being increasingly utilized to enhance the efficiency and accuracy of regulatory submissions. These technologies enable companies to analyze vast amounts of data, identify potential compliance issues, and streamline the approval process. As the industry embraces digital transformation, the demand for outsourcing regulatory affairs is expected to rise, as specialized firms equipped with these technologies can offer enhanced services. This trend indicates a shift towards more efficient regulatory practices, suggesting that the Medical Device Regulatory Affairs Outsourcing Market will continue to evolve in response to technological advancements.