Focus on Cost Efficiency
Cost efficiency remains a pivotal driver in the healthcare regulatory-affairs-outsourcing market. Japanese companies are increasingly seeking to reduce operational costs while maintaining compliance with stringent regulations. Outsourcing regulatory affairs allows organizations to leverage the expertise of specialized firms, which can lead to significant savings. Reports suggest that companies can reduce their regulatory compliance costs by up to 30% through outsourcing. This financial incentive is compelling, especially for small to medium-sized enterprises that may lack the resources to maintain in-house regulatory teams. As a result, the trend towards outsourcing is likely to continue, further propelling the growth of the healthcare regulatory-affairs-outsourcing market.
Emphasis on Quality Assurance
Quality assurance is becoming increasingly critical in the healthcare regulatory-affairs-outsourcing market. As regulatory scrutiny intensifies, companies are prioritizing compliance with quality standards to avoid costly penalties and reputational damage. Outsourcing partners are expected to provide robust quality assurance processes, which can enhance the overall compliance framework of the organizations they serve. In Japan, the emphasis on quality has led to a rise in demand for outsourcing services that specialize in quality management systems. This trend is likely to continue, as companies recognize the value of maintaining high-quality standards in their regulatory submissions, thus driving growth in the healthcare regulatory-affairs-outsourcing market.
Increasing Complexity of Regulations
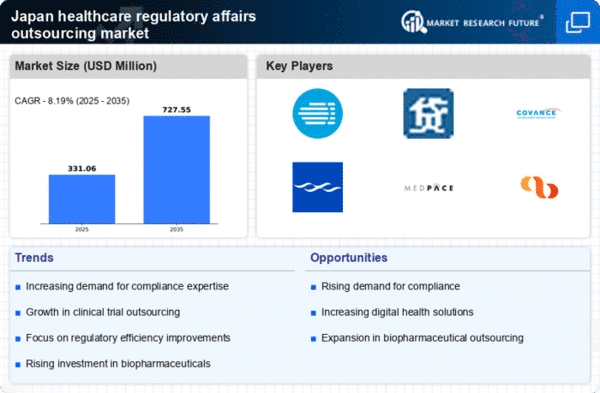
The healthcare regulatory-affairs-outsourcing market is experiencing a surge in demand due to the increasing complexity of regulations in Japan. As the regulatory landscape evolves, companies are finding it challenging to navigate the myriad of compliance requirements. This complexity necessitates specialized knowledge and expertise, prompting organizations to outsource regulatory affairs to ensure adherence to local laws and international standards. The Japanese market has seen a notable rise in regulatory submissions, with an increase of approximately 15% in the last year alone. This trend indicates a growing reliance on outsourcing partners who can provide the necessary regulatory expertise, thereby driving growth in the healthcare regulatory-affairs-outsourcing market.
Rising Demand for Faster Market Access
In Japan, the demand for faster market access is driving the healthcare regulatory-affairs-outsourcing market. Companies are under pressure to expedite the approval process for new drugs and medical devices to remain competitive. The average time for regulatory approval has been a concern, with recent data indicating that it can take up to 18 months for new products to receive clearance. Outsourcing regulatory affairs can streamline this process, as specialized firms often have established relationships with regulatory bodies and a deep understanding of the approval landscape. This capability to facilitate quicker market entry is increasingly attractive to companies, thereby enhancing the growth prospects of the healthcare regulatory-affairs-outsourcing market.
Technological Integration in Regulatory Processes
The integration of technology into regulatory processes is a significant driver of the healthcare regulatory-affairs-outsourcing market. In Japan, advancements in digital tools and platforms are transforming how regulatory affairs are managed. Companies are increasingly adopting electronic submission systems and data analytics to enhance efficiency and accuracy in compliance reporting. This technological shift not only streamlines the regulatory process but also reduces the likelihood of errors, which can be costly. As organizations seek to leverage these technological advancements, the demand for outsourcing services that can provide these capabilities is expected to rise, thereby propelling the growth of the healthcare regulatory-affairs-outsourcing market.
















Leave a Comment