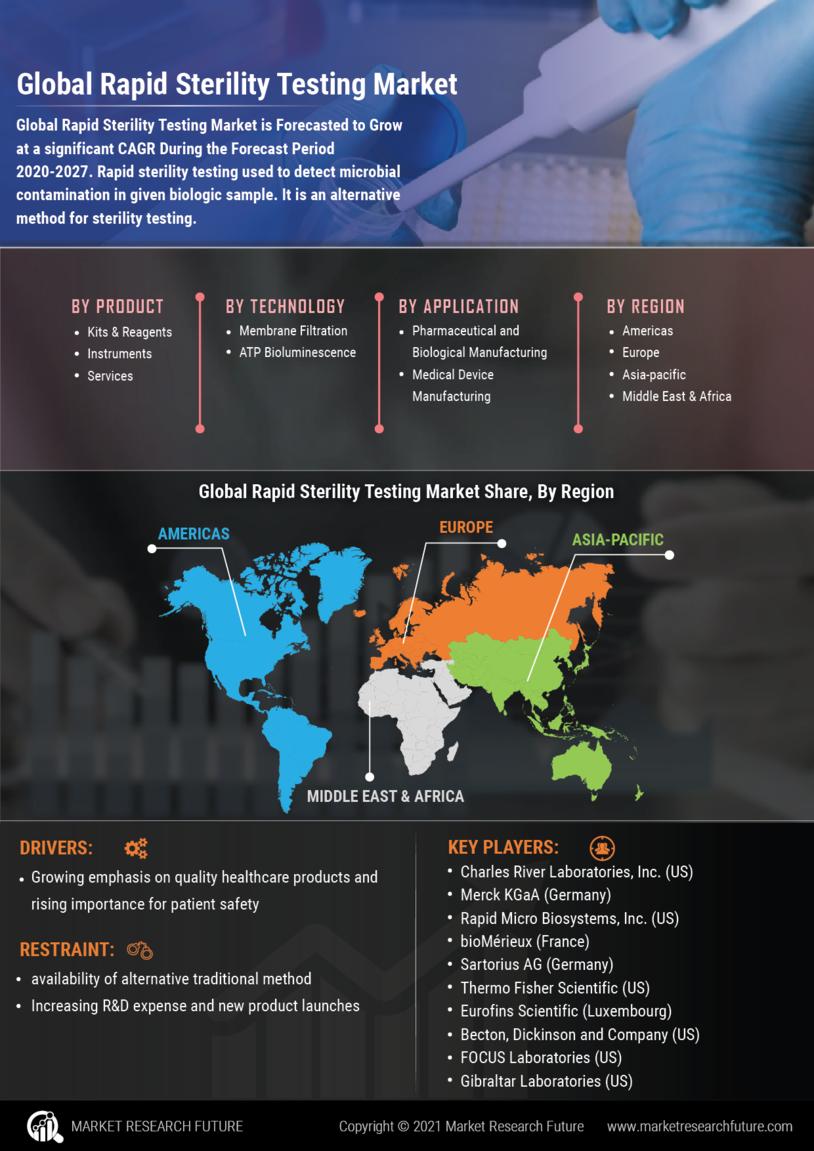
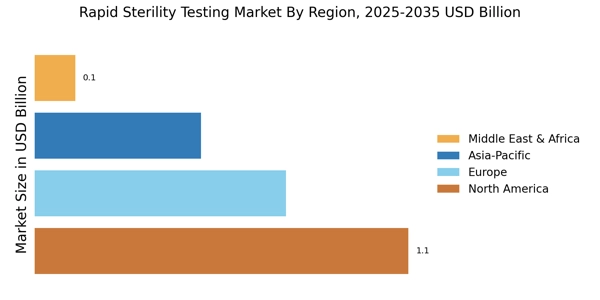
Expansion of Healthcare Infrastructure
The expansion of healthcare infrastructure is a notable driver for the Rapid Sterility Testing Market. As countries invest in improving their healthcare systems, the demand for advanced testing solutions is expected to grow. New healthcare facilities, particularly in emerging markets, are increasingly incorporating rapid sterility testing into their quality control processes. This trend is indicative of a broader movement towards modernization and efficiency in healthcare delivery. The establishment of new laboratories and testing centers is likely to further stimulate market growth, as these facilities seek to implement the latest testing technologies.
Growing Awareness of Infection Control
The Rapid Sterility Testing Market is also benefiting from the heightened awareness surrounding infection control practices. As healthcare facilities and pharmaceutical companies prioritize infection prevention, the demand for rapid sterility testing solutions is on the rise. This awareness is particularly pronounced in settings where sterile products are critical, such as surgical environments and drug manufacturing. The market is likely to see an increase in adoption rates of rapid testing methods as organizations strive to enhance their infection control protocols, thereby ensuring patient safety and product reliability.
Regulatory Pressure for Faster Testing
Regulatory bodies are imposing stricter guidelines on sterility testing, which is a key driver for the Rapid Sterility Testing Market. These regulations aim to ensure that pharmaceutical and biopharmaceutical products meet safety standards before reaching consumers. Consequently, companies are compelled to adopt rapid testing methods to comply with these regulations. The increasing emphasis on patient safety and product quality is likely to drive the market, as organizations seek to minimize the time taken for testing without compromising on accuracy. This trend indicates a shift towards more efficient testing methodologies in the industry.
Increased Demand for Biopharmaceuticals
The Rapid Sterility Testing Market is significantly influenced by the rising demand for biopharmaceuticals. As the biopharmaceutical sector expands, the need for stringent sterility testing becomes paramount to ensure product safety and efficacy. This demand is further fueled by the increasing prevalence of chronic diseases and the growing focus on personalized medicine. According to industry reports, the biopharmaceutical market is expected to reach USD 500 billion by 2026, thereby propelling the need for rapid sterility testing solutions to meet regulatory requirements and maintain product integrity.
Technological Advancements in Rapid Sterility Testing
The Rapid Sterility Testing Market is experiencing a surge in technological advancements that enhance testing efficiency and accuracy. Innovations such as automated systems and real-time monitoring are becoming increasingly prevalent. These advancements not only reduce the time required for sterility testing but also improve the reliability of results. For instance, the integration of molecular techniques, such as PCR, allows for quicker detection of microbial contamination. As a result, the market is projected to grow at a compound annual growth rate of approximately 10% over the next few years, driven by the need for faster and more reliable testing solutions.