Rising Prevalence of Epilepsy
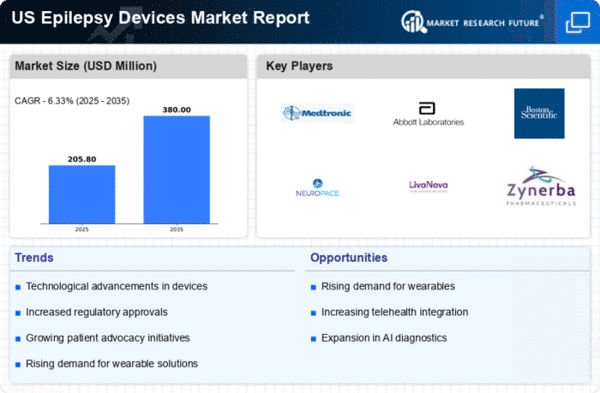
The increasing prevalence of epilepsy in the US is a primary driver for the epilepsy devices market. According to the CDC, approximately 3.4 million people in the US are living with epilepsy, which translates to about 1.2% of the population. This growing patient base necessitates the development and adoption of advanced epilepsy devices, such as seizure detection systems and neurostimulation devices. As awareness of epilepsy rises, healthcare providers are more likely to recommend these devices, thereby expanding the market. Furthermore, the aging population is likely to contribute to a higher incidence of epilepsy, as age is a significant risk factor. Consequently, the epilepsy devices market is poised for growth as more individuals seek effective management solutions for their condition.
Regulatory Support for Device Approval
Regulatory support for device approval is a critical driver of the epilepsy devices market. The FDA has streamlined the approval process for certain epilepsy devices, allowing for faster access to innovative treatments. This regulatory environment encourages manufacturers to invest in research and development, knowing that their products may reach the market more quickly. The introduction of the Breakthrough Devices Program has expedited the approval of devices that offer advantages over existing treatments.. As a result, patients benefit from timely access to new technologies that can improve their quality of life. This supportive regulatory framework is likely to foster growth in the epilepsy devices market, as companies are motivated to bring their innovations to fruition.
Increased Funding for Epilepsy Research
Increased funding for epilepsy research is significantly impacting the epilepsy devices market. Government initiatives and private sector investments are focusing on developing new therapies and devices for epilepsy management. For instance, the National Institutes of Health (NIH) has allocated substantial resources to epilepsy research, which has led to breakthroughs in device technology. This financial support encourages innovation and the introduction of novel devices into the market. As research progresses, new findings may lead to improved device efficacy and safety, which could enhance patient outcomes. Consequently, the influx of funding is likely to stimulate growth in the epilepsy devices market, as manufacturers strive to meet the evolving needs of patients and healthcare providers.
Technological Innovations in Device Design
Technological innovations play a crucial role in shaping the epilepsy devices market. Recent advancements in wearable technology, such as smartwatches and portable EEG monitors, have enhanced the ability to monitor seizures in real-time. These devices often utilize sophisticated algorithms to analyze brain activity, providing valuable data to both patients and healthcare providers. The integration of artificial intelligence and machine learning into these devices is expected to improve their accuracy and reliability. As a result, patients are more likely to adopt these innovative solutions, driving market growth. Moreover, the development of non-invasive stimulation devices offers new treatment options, further expanding the epilepsy devices market. The continuous evolution of technology indicates a promising future for device manufacturers and patients alike..
Growing Awareness and Education Initiatives
Growing awareness and education initiatives regarding epilepsy are driving the epilepsy devices market. Campaigns aimed at educating the public about epilepsy and its management have increased the visibility of available devices. Organizations such as the Epilepsy Foundation are actively promoting awareness, which helps to destigmatize the condition and encourages individuals to seek treatment. As more patients become informed about the benefits of using epilepsy devices, the demand for these products is likely to rise. Additionally, healthcare professionals are increasingly recognizing the importance of discussing device options with patients, further propelling market growth. This heightened awareness and education are essential for fostering a supportive environment for individuals living with epilepsy.