Growing Geriatric Population
The Epilepsy Devices Market is also impacted by the growing geriatric population, which is more susceptible to epilepsy. As the global population ages, the incidence of epilepsy is expected to rise, leading to an increased demand for effective management devices. Older adults often experience different seizure types and may require specialized devices that cater to their unique needs. The market is projected to expand as healthcare providers seek to address the challenges associated with managing epilepsy in this demographic. Additionally, the integration of user-friendly technology in devices designed for older patients is likely to enhance adherence and improve quality of life, further driving market growth.
Rising Awareness and Advocacy
The Epilepsy Devices Market is benefiting from rising awareness and advocacy efforts aimed at educating the public about epilepsy. Organizations and advocacy groups are actively working to reduce stigma and promote understanding of the condition, which is crucial for improving patient access to treatment options. Increased awareness has led to a higher demand for epilepsy devices, as more individuals seek effective management solutions. According to recent surveys, nearly 70% of people with epilepsy can achieve seizure control with appropriate treatment, highlighting the importance of accessible devices. This growing recognition of epilepsy as a significant health issue is likely to propel the market forward, as more patients and healthcare providers prioritize effective management strategies.
Government Initiatives and Funding
The Epilepsy Devices Market is significantly influenced by government initiatives and funding aimed at improving epilepsy care. Various governments are investing in research and development to foster innovation in medical devices for epilepsy management. These initiatives often include grants and subsidies for companies developing cutting-edge technologies, which can enhance the availability of effective devices. For instance, funding for clinical trials of new epilepsy devices is crucial for validating their efficacy and safety. As governments recognize the burden of epilepsy on public health, increased financial support is likely to stimulate market growth and encourage the development of novel solutions that address the needs of patients.
Increased Focus on Personalized Medicine
The Epilepsy Devices Market is witnessing a notable shift towards personalized medicine, which tailors treatment plans to individual patient needs. This trend is largely influenced by advancements in genetic research and biomarker identification, enabling healthcare providers to customize therapies based on a patient's unique genetic profile. As a result, devices that can adapt to specific seizure types and patient responses are becoming more prevalent. The market is expected to grow as healthcare systems increasingly adopt personalized approaches, potentially leading to improved treatment adherence and outcomes. This focus on individualized care is likely to enhance the overall effectiveness of epilepsy management, thereby driving demand for innovative devices.
Technological Advancements in Epilepsy Devices
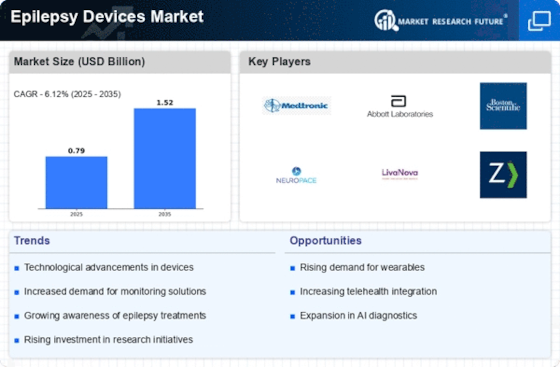
The Epilepsy Devices Market is experiencing a surge in technological advancements, which are pivotal in enhancing the efficacy of treatment options. Innovations such as wearable devices, implantable neurostimulators, and advanced monitoring systems are being developed to provide real-time data and improve patient outcomes. For instance, the integration of artificial intelligence in seizure prediction algorithms is transforming how healthcare providers manage epilepsy. The market for epilepsy devices is projected to reach approximately USD 5 billion by 2026, driven by these technological innovations. Furthermore, the development of mobile applications that assist in tracking seizure activity is likely to empower patients and caregivers, thereby fostering a more proactive approach to epilepsy management.