Rising Incidence of Epilepsy
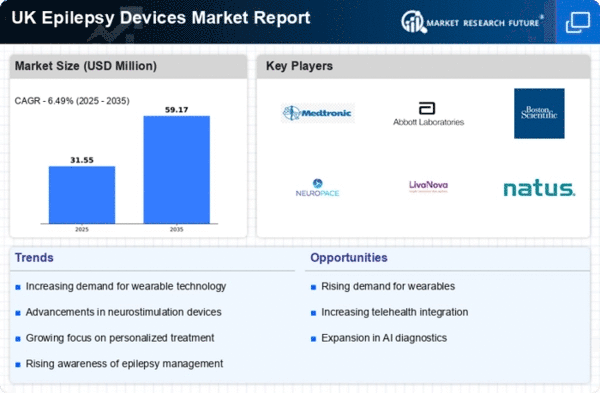
The increasing prevalence of epilepsy in the UK is a crucial driver for the epilepsy devices market. Recent estimates suggest that approximately 600,000 individuals in the UK are living with epilepsy, which translates to about 1 in 100 people. This rising incidence necessitates the development and adoption of advanced devices for monitoring and managing seizures. As the population ages, the incidence of epilepsy is expected to rise, further propelling the demand for innovative devices. The epilepsy devices market is likely to benefit from this trend. Healthcare providers seek effective solutions to improve patient outcomes and reduce healthcare costs associated with epilepsy management.
Government Initiatives and Funding
Government initiatives aimed at improving healthcare access and funding for epilepsy research are vital drivers for the epilepsy devices market. The UK government has allocated substantial resources to enhance epilepsy care, including funding for innovative device development. This financial support encourages manufacturers to invest in research and development, leading to the introduction of cutting-edge devices. Additionally, public health campaigns aimed at raising awareness about epilepsy are likely to increase the demand for effective management solutions. As a result, the epilepsy devices market is expected to expand, driven by both public and private sector investments.
Increased Focus on Patient-Centric Care
The growing emphasis on patient-centric care in the UK healthcare system is a significant driver for the epilepsy devices market. Healthcare providers are increasingly prioritizing the needs and preferences of patients, leading to the development of devices that enhance user experience and engagement. This shift is reflected in the design of epilepsy devices that are more user-friendly and accessible. Moreover, the integration of patient feedback into the development process is likely to result in more effective solutions. As a consequence, the epilepsy devices market is expected to thrive as it aligns with the broader trend of improving patient outcomes through tailored healthcare solutions.
Growing Demand for Personalized Medicine
The shift towards personalized medicine in the treatment of epilepsy is influencing the epilepsy devices market. Patients are increasingly seeking tailored solutions that cater to their specific needs, which has led to the development of customized devices. This trend is supported by advancements in genetic research and data analytics, enabling healthcare providers to offer more precise treatment options. The epilepsy devices market is likely to see growth as manufacturers respond to this demand by creating devices that can be adapted to individual patient profiles, thereby improving treatment efficacy and patient satisfaction.
Technological Innovations in Device Design
Technological advancements in the design and functionality of epilepsy devices are significantly influencing the market. Innovations such as wearable devices, implantable monitors, and mobile applications are enhancing the ability to track seizure activity in real-time. For instance, the introduction of devices that utilize artificial intelligence for predictive analytics is transforming patient care. The epilepsy devices market is projected to grow as these technologies become more accessible and affordable. Furthermore, the integration of telemedicine with these devices allows for remote monitoring, which is particularly beneficial in the UK, where healthcare access can vary by region.