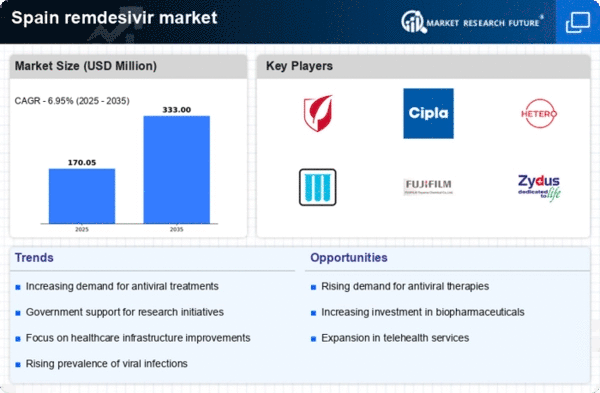
Rising Demand for Antiviral Treatments
The remdesivir market in Spain is experiencing a notable increase in demand for antiviral treatments. This trend is driven by a growing awareness of viral infections and the need for effective therapeutic options. As healthcare providers and patients seek reliable solutions, remdesivir has emerged as a preferred choice due to its efficacy against certain viral pathogens. Market data indicates that the demand for antiviral medications has surged by approximately 15% in recent years, reflecting a shift in treatment paradigms. The remdesivir market is likely to benefit from this rising demand, as healthcare systems prioritize the availability of effective antiviral therapies to combat emerging viral threats.
Advancements in Pharmaceutical Research
Advancements in pharmaceutical research are propelling the remdesivir market forward in Spain. Ongoing research initiatives are focused on optimizing the formulation and delivery of remdesivir, enhancing its therapeutic efficacy. The Spanish pharmaceutical sector has seen a surge in research funding, with an increase of 20% in the last year, aimed at developing innovative antiviral treatments. These advancements not only improve patient outcomes but also contribute to the overall growth of the remdesivir market. As new research findings emerge, the market is likely to witness an influx of improved formulations and treatment protocols, further solidifying remdesivir's position in the antiviral landscape.
Investment in Healthcare Infrastructure
Investment in healthcare infrastructure in Spain is a critical driver for the remdesivir market. The Spanish government has been allocating substantial resources to enhance healthcare facilities and improve access to essential medications. This investment is expected to facilitate the distribution and availability of remdesivir, ensuring that patients have timely access to this antiviral treatment. Recent reports suggest that healthcare spending in Spain has increased by 10% over the past year, indicating a commitment to strengthening the healthcare system. As infrastructure improves, the remdesivir market is poised to expand, providing greater access to this vital medication for patients in need.
Increased Collaboration Among Stakeholders
Increased collaboration among stakeholders is a vital driver for the remdesivir market in Spain. Pharmaceutical companies, healthcare providers, and government agencies are working together to enhance the accessibility and distribution of remdesivir. This collaborative approach aims to streamline the supply chain and ensure that patients receive timely treatment. Recent initiatives have led to partnerships that focus on improving logistics and distribution networks, which are crucial for the effective delivery of antiviral medications. As collaboration continues to strengthen, the remdesivir market is expected to benefit from improved access and availability, ultimately enhancing patient care in Spain.
Growing Focus on Infectious Disease Management
The remdesivir market is significantly influenced by the growing focus on infectious disease management in Spain. With an increasing incidence of viral infections, healthcare authorities are prioritizing the development and distribution of effective antiviral therapies. Remdesivir, recognized for its potential in treating specific viral infections, is likely to play a pivotal role in this strategy. Market analysis reveals that investments in infectious disease management have risen by 12% in the last year, underscoring the urgency to address these health challenges. This heightened focus on managing infectious diseases is expected to drive the growth of the remdesivir market as healthcare providers seek effective treatment options.