Leading market players are investing heavily in research and development in order to expand their product lines, which will help the Remdesivir Market (COVID 19) market, grow even more. Market participants are also undertaking a variety of strategic activities to expand their footprint, with important market developments including new product launches, contractual agreements, mergers and acquisitions, higher investments, and collaboration with other organizations. To expand and survive in a more competitive and rising market climate, Remdesivir Market (COVID 19) industry must offer cost-effective items.
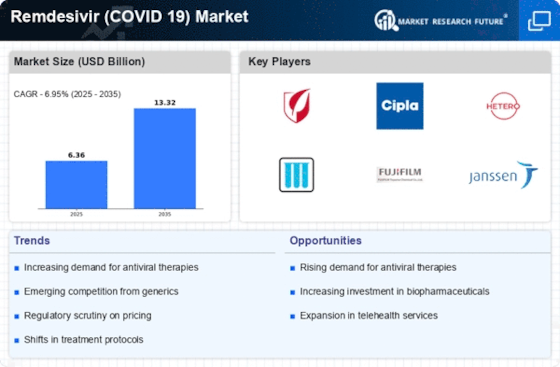
Manufacturing locally to minimize operational costs is one of the key business tactics used by manufacturers in the Remdesivir Market (COVID 19) industry to benefit clients and increase the market sector. In recent years, the Remdesivir Market (COVID 19) industry has offered some of the most significant advantages to medicine. Major players in the Remdesivir Market (COVID 19) market, including Gilead Sciences, Inc. (US), Mylan (US), Cipla (India), Hetero Labs (India), and Jubilant Life Sciences (India), and others, are attempting to increase market demand by investing in research and development operations.
A pharmaceutical firm called Cipla Ltd. (Cipla) produces and distributes branded medications, generic drugs, and active pharmaceutical ingredients (APIs). It provides products for conditions like cardiovascular and paediatric illnesses, dermatological and cosmetological issues, HIV/AIDS, diabetes, hepatitis, infectious diseases, critical care, neurological issues, ophthalmic, cancer, malaria, respiratory and urological issues, osteoporosis, and women's health. The corporation also engages in biosimilar and consumer healthcare. In addition to producing metered-dose inhaler equipment, spacers, and associated devices, Cipla also carries out research and development to create new drugs and medication delivery methods.
The corporation operates in a number of important regulated and emerging markets, including India, the US, Canada, and South Africa. The headquarters of Cipla are in Mumbai, Maharashtra, India.
A biopharmaceutical company with a research foundation is Gilead Sciences Inc. (Gilead). It is involved in the discovery, development, and commercialization of medications for the treatment of cancer, inflammatory illnesses of the liver, cardiovascular, haematological, and respiratory systems, as well as HIV infection. Through affiliates and distributors in Europe, the Americas, Asia-Pacific, the Middle East, and Africa, the corporation offers its goods. In addition to other places, it has production operations in Cork, Ireland, Foster City, San Dimas, and Oceanside, California. To create new medicines, the company collaborates with academic institutions, medical research centres, and major international pharmaceutical companies.
The US city of Foster City is where Gilead is based.