Rising Awareness of Viral Infections
There is a growing awareness of viral infections among the Japanese population, which is influencing the remdesivir market. Increased public education campaigns and media coverage regarding the risks associated with viral diseases have led to heightened demand for effective treatments. This awareness is likely to drive healthcare professionals to prescribe remdesivir more frequently, as patients become more informed about their treatment options. Additionally, the rise in health literacy is prompting patients to seek timely medical intervention, further propelling the market. As awareness continues to grow, the remdesivir market may experience a corresponding increase in utilization rates.
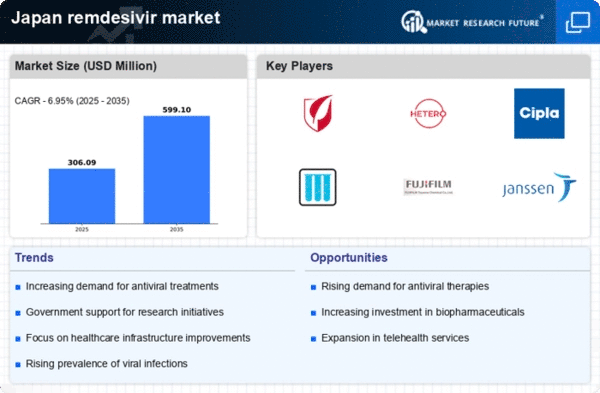
Increasing Demand for Antiviral Treatments
The rising prevalence of viral infections in Japan has led to an increasing demand for antiviral treatments, including those in the remdesivir market. As healthcare providers seek effective solutions to combat viral outbreaks, the market for remdesivir is expected to expand. Recent data indicates that the antiviral drug market in Japan is projected to grow at a CAGR of approximately 8% over the next five years. This growth is driven by the need for rapid response to emerging viral threats, which positions remdesivir as a critical component in the treatment arsenal. Furthermore, the Japanese government's focus on enhancing healthcare infrastructure and access to innovative therapies is likely to bolster the remdesivir market, ensuring that patients receive timely and effective treatment.
Government Initiatives for Pandemic Preparedness
The Japanese government has implemented various initiatives aimed at pandemic preparedness, which significantly impact the remdesivir market. These initiatives include funding for research, stockpiling antiviral medications, and establishing rapid response protocols. The government’s commitment to ensuring a robust healthcare system is evident in its allocation of approximately ¥100 billion for pandemic preparedness in recent budgets. Such measures not only enhance the availability of remdesivir but also foster a conducive environment for its market growth. As Japan continues to prioritize public health and safety, the remdesivir market is expected to thrive, driven by these proactive governmental strategies.
Technological Advancements in Drug Delivery Systems
Technological innovations in drug delivery systems are playing a pivotal role in the remdesivir market in Japan. Enhanced delivery mechanisms, such as nanotechnology and targeted delivery, are improving the efficacy and safety profiles of antiviral medications. These advancements not only facilitate better patient outcomes but also increase the overall market potential for remdesivir. For instance, the integration of smart delivery systems can optimize dosing regimens, potentially leading to improved adherence and therapeutic effectiveness. As the Japanese pharmaceutical industry continues to invest in research and development, the remdesivir market is likely to benefit from these technological enhancements, which may result in a more favorable competitive landscape.
Collaborative Research Efforts in the Pharmaceutical Sector
Collaborative research efforts among pharmaceutical companies, academic institutions, and government agencies are fostering innovation within the remdesivir market. These partnerships are essential for accelerating the development of new formulations and treatment protocols. In Japan, several collaborative initiatives have been launched, focusing on enhancing the efficacy of antiviral therapies. Such collaborations not only pool resources but also facilitate knowledge sharing, which can lead to breakthroughs in treatment options. As these partnerships continue to evolve, they are likely to create a more dynamic and competitive environment for the remdesivir market, ultimately benefiting patients and healthcare providers alike.