Government Initiatives and Funding
Government initiatives in France play a crucial role in shaping the remdesivir market. Increased funding for research and development of antiviral drugs, including remdesivir, is evident. The French government has allocated substantial resources to support pharmaceutical innovation, which is expected to enhance the availability of remdesivir. Additionally, public health campaigns aimed at educating healthcare professionals about the benefits of antiviral treatments are likely to drive market growth. With an estimated €50 million earmarked for antiviral research in the upcoming fiscal year, the remdesivir market stands to benefit significantly from these initiatives, fostering a conducive environment for market expansion.
Rising Demand for Antiviral Treatments
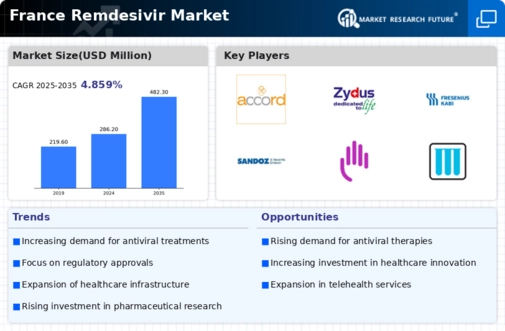
The remdesivir market in France is experiencing a notable increase in demand for antiviral treatments. This surge is driven by a heightened awareness of viral infections and the need for effective therapeutic options. As healthcare providers and patients seek reliable solutions, the market is projected to grow at a CAGR of approximately 8% over the next few years. The French healthcare system is increasingly prioritizing antiviral medications, which is likely to bolster the remdesivir market. Furthermore, the ongoing research and development efforts aimed at enhancing the efficacy of antiviral drugs contribute to this rising demand, indicating a robust future for the remdesivir market in France.
Advancements in Pharmaceutical Technology
Technological advancements in the pharmaceutical sector are influencing the remdesivir market in France. Innovations in drug formulation and delivery systems are enhancing the effectiveness of remdesivir, making it more appealing to healthcare providers. The integration of advanced manufacturing techniques is expected to reduce production costs, potentially leading to lower prices for consumers. As a result, the remdesivir market may witness increased accessibility and adoption among healthcare facilities. Furthermore, the introduction of novel drug delivery methods could improve patient compliance, thereby positively impacting the overall market dynamics in France.
Collaboration Between Public and Private Sectors
Collaboration between public and private sectors is emerging as a key driver for the remdesivir market in France. Partnerships between government agencies and pharmaceutical companies are fostering innovation and expediting the development of antiviral treatments. These collaborations are likely to enhance the research capabilities and distribution networks for remdesivir, ensuring its availability across various healthcare settings. With an estimated €30 million invested in joint research initiatives, the synergy between public and private entities is expected to yield significant advancements in the remdesivir market. This collaborative approach may also facilitate faster regulatory approvals, further benefiting the market.
Growing Focus on Infectious Disease Preparedness
The remdesivir market is being propelled by a growing focus on infectious disease preparedness in France. The government and healthcare institutions are increasingly recognizing the importance of having effective antiviral treatments readily available. This proactive approach is likely to lead to strategic stockpiling of remdesivir, ensuring that it is accessible during outbreaks. The French healthcare system's commitment to enhancing its response capabilities is expected to create a favorable environment for the remdesivir market. As a result, investments in antiviral therapies are anticipated to rise, further solidifying the market's position in the healthcare landscape.