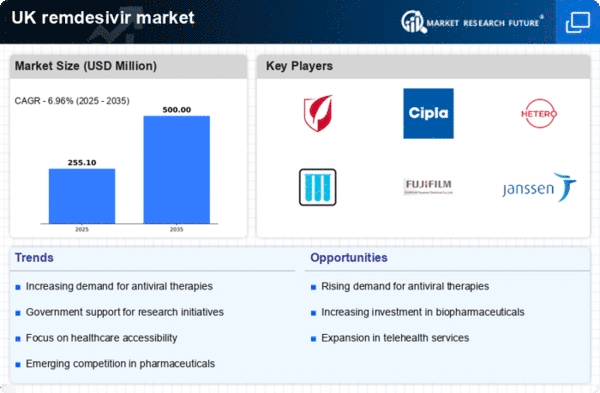
Rising Healthcare Expenditure
The remdesivir market in the UK is likely to benefit from the increasing healthcare expenditure observed in recent years. The UK government has been allocating substantial funds to enhance healthcare services, which includes investments in antiviral treatments. In 2025, healthcare spending is projected to reach approximately £200 billion, reflecting a growth of around 5% from the previous year. This financial commitment may facilitate greater access to remdesivir, thereby driving its demand. Furthermore, as healthcare budgets expand, hospitals and clinics are more inclined to stock essential antiviral medications, including remdesivir, to ensure preparedness for potential outbreaks. This trend indicates a robust market environment for remdesivir, as healthcare providers prioritize effective treatment options to manage viral infections.
Emerging Variants and Treatment Needs
The emergence of new viral variants continues to pose challenges for public health in the UK, which may drive the demand for remdesivir in the market. As variants evolve, the need for effective antiviral treatments becomes increasingly critical. In 2025, health authorities report a rise in cases attributed to new strains, necessitating the availability of robust treatment options. This situation suggests that remdesivir could play a pivotal role in managing infections caused by these variants. The remdesivir market may experience growth as healthcare systems adapt to the changing landscape of viral infections. Additionally, ongoing research into the efficacy of remdesivir against emerging variants may further bolster its demand, as healthcare providers seek reliable solutions to combat these evolving threats.
Government Support and Funding Initiatives
Government support and funding initiatives are likely to play a crucial role in shaping the remdesivir market in the UK. The UK government has been actively investing in research and development for antiviral medications, including remdesivir. In 2025, funding for antiviral research is expected to exceed £50 million, aimed at enhancing treatment options and ensuring availability during health crises. This financial backing may encourage pharmaceutical companies to increase production and distribution of remdesivir, thereby improving access for healthcare providers. Furthermore, government initiatives to streamline regulatory processes for antiviral drugs could expedite the introduction of remdesivir into the market, fostering a more competitive environment. Such support indicates a proactive approach to addressing public health needs, which may ultimately benefit the remdesivir market.
Increased Awareness of Antiviral Therapies
Public awareness regarding antiviral therapies appears to be on the rise in the UK, which could positively influence the remdesivir market. Educational campaigns and media coverage have contributed to a better understanding of the importance of antiviral treatments in managing viral infections. As patients become more informed about available options, the demand for effective medications like remdesivir is likely to increase. In 2025, surveys indicate that approximately 70% of the population is aware of antiviral treatments, a notable increase from previous years. This heightened awareness may lead to more patients seeking prescriptions for remdesivir, thereby expanding its market presence. Consequently, healthcare providers may respond by increasing their stock of remdesivir, further solidifying its position in the antiviral treatment landscape.
Collaboration Between Public and Private Sectors
Collaboration between public and private sectors is emerging as a significant driver for the remdesivir market in the UK. Partnerships between government agencies and pharmaceutical companies are fostering innovation and improving the supply chain for antiviral treatments. In 2025, several collaborative projects are underway, focusing on enhancing the production and distribution of remdesivir. These partnerships may lead to increased efficiency in bringing remdesivir to market, ensuring that healthcare providers have timely access to this essential medication. Additionally, joint efforts in research and development could yield new formulations or delivery methods for remdesivir, further expanding its therapeutic potential. This collaborative approach suggests a dynamic market environment, where shared resources and expertise contribute to the growth of the remdesivir market.