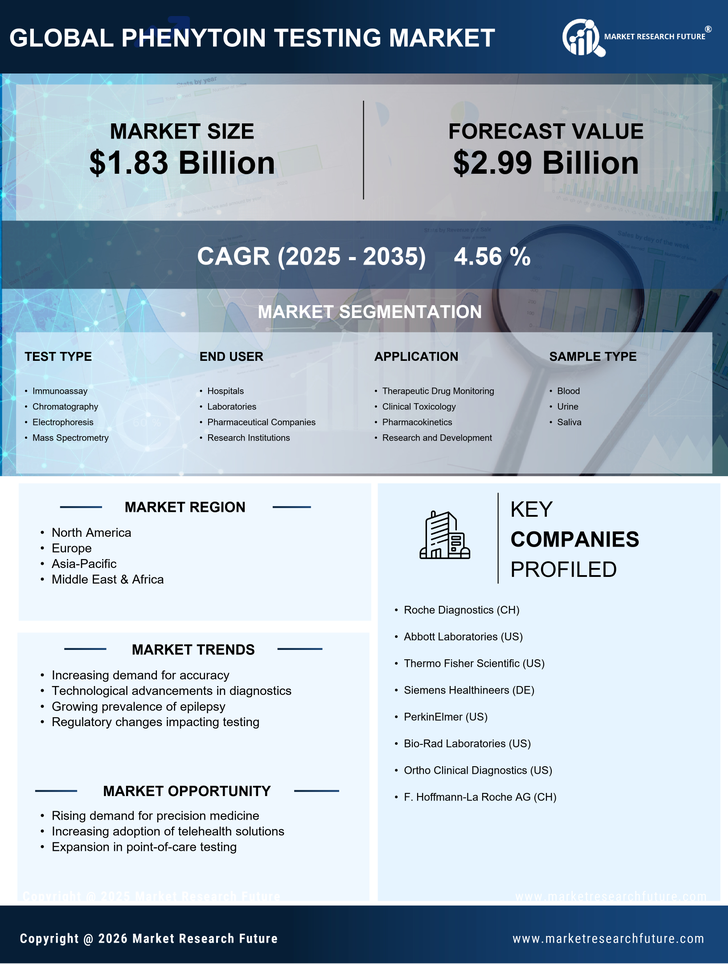
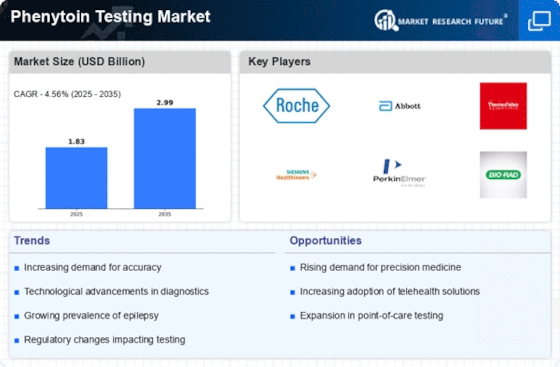
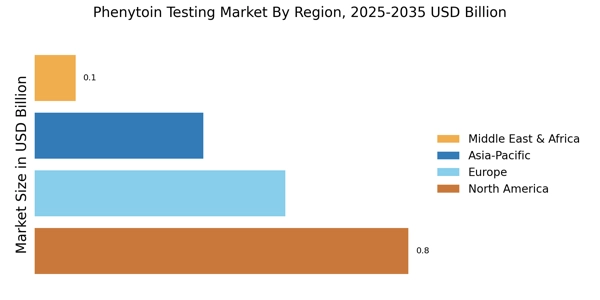
Rising Incidence of Epilepsy
The increasing prevalence of epilepsy worldwide is a primary driver for the Phenytoin Testing Market. As epilepsy affects approximately 50 million people globally, the demand for effective management and monitoring of this condition has surged. Phenytoin, a widely used antiepileptic drug, necessitates regular testing to ensure therapeutic levels are maintained. This growing patient population is likely to propel the need for reliable testing solutions, thereby expanding the Phenytoin Testing Market. Furthermore, as healthcare providers emphasize personalized medicine, the requirement for precise monitoring of drug levels becomes even more critical, suggesting a robust growth trajectory for the market.
Regulatory Support for Drug Testing
Regulatory bodies are increasingly emphasizing the importance of drug testing, which positively impacts the Phenytoin Testing Market. Guidelines and recommendations from health authorities advocate for routine monitoring of antiepileptic drugs, including phenytoin, to ensure patient safety and treatment efficacy. This regulatory support encourages healthcare providers to adopt testing protocols, thereby driving market growth. Additionally, as regulations evolve to enhance quality control in laboratories, the demand for reliable phenytoin testing solutions is likely to increase. The Phenytoin Testing Market stands to benefit from these regulatory frameworks, which aim to improve patient care and therapeutic outcomes.
Expansion of Healthcare Infrastructure
The ongoing expansion of healthcare infrastructure in various regions is a crucial driver for the Phenytoin Testing Market. As healthcare facilities grow and improve, the capacity for diagnostic testing, including phenytoin levels, is enhanced. This expansion is particularly evident in emerging markets, where investments in healthcare are increasing. Enhanced access to testing services allows for better management of epilepsy and other conditions requiring phenytoin, thereby boosting the demand for testing solutions. Furthermore, as healthcare systems prioritize comprehensive patient care, the Phenytoin Testing Market is likely to experience sustained growth, reflecting the broader trends in healthcare development.
Advancements in Diagnostic Technologies
Technological innovations in diagnostic testing are significantly influencing the Phenytoin Testing Market. The introduction of advanced analytical techniques, such as high-performance liquid chromatography (HPLC) and mass spectrometry, enhances the accuracy and efficiency of phenytoin level assessments. These advancements not only improve patient outcomes but also streamline laboratory workflows, making testing more accessible. As healthcare facilities increasingly adopt these cutting-edge technologies, the Phenytoin Testing Market is poised for expansion. Moreover, the integration of automation in laboratories is likely to reduce turnaround times, further driving the demand for phenytoin testing solutions.
Growing Awareness of Therapeutic Drug Monitoring
There is a notable increase in awareness regarding therapeutic drug monitoring (TDM) among healthcare professionals and patients, which serves as a significant driver for the Phenytoin Testing Market. TDM is essential for optimizing drug therapy, particularly for medications with narrow therapeutic indices like phenytoin. As clinicians recognize the importance of monitoring drug levels to prevent toxicity and ensure efficacy, the demand for phenytoin testing is expected to rise. This trend is supported by various studies indicating that effective TDM can lead to improved patient outcomes, thereby reinforcing the necessity for robust testing solutions within the Phenytoin Testing Market.