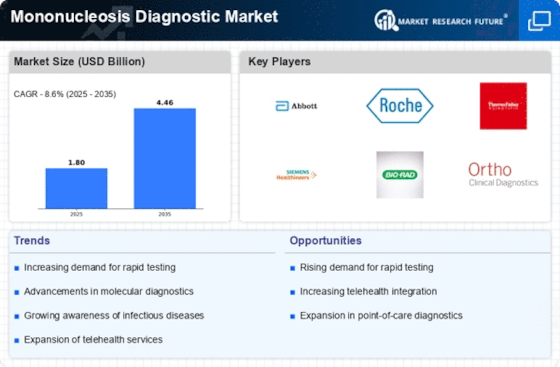
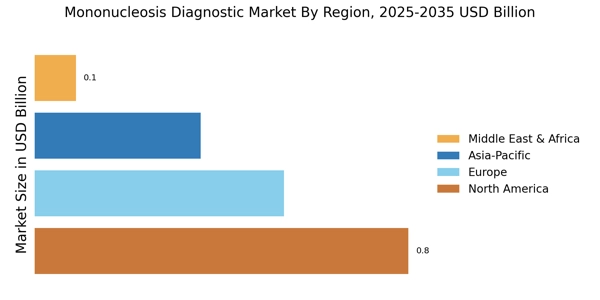
By region, the study provides market insights into North America, Europe, Asia-Pacific, and Rest of the World. The anticipated expansion of the Mononucleosis Diagnostic Market in North America can be attributed to several key factors. More promising factors are the relatively well-developed healthcare infrastructure and high adoption of advanced diagnosis techniques, which are the region's key points to higher growth. U.S. healthcare spending grew 4.1 percent in 2022, reaching $4.5 trillion or $13,493 per person.
As a share of the nation's Gross Domestic Product, health spending accounted for 17.3 percent. This region segment`s mononucleosis diagnostic market is being driven by two major factors: the rising need for immediate and accurate diagnosis, including the high public uptake of the necessity for fast diagnosis and treatment.
Further, the major countries studied in the market report are the US, Canada, Germany, France, the UK, Italy, Spain, China, Japan, India, Australia, South Korea, and Brazil.
Figure 2: MONONUCLEOSIS DIAGNOSTIC MARKET SHARE BY REGION 2023 (USD Billion)
Europe's Mononucleosis Diagnostic Market accounts for the second-largest market share. One virus in particular that causes mononucleosis, the most popular disease known as "the kissing disease" or "mono," is called Epstein-Barr Virus (EBV). In 2022, the number of infectious mononucleosis cases in Spain amounted to nearly 26,400, up from 18,700 cases reported a year earlier. The highest number of mononucleosis cases in the European country was registered in 2016, after reaching approximately 46,300 thousand infections. This influenza virus has increased in prevalence across Europe, causing more and more demand for diagnostic tests.
Further, the German Mononucleosis Diagnostic Market held the largest market share, and the UK Mononucleosis Diagnostic Market was the fastest-growing market in the European region.
The Asia-Pacific Mononucleosis Diagnostic Market is expected to grow at the fastest CAGR from 2024 to 2032. As per the United Nations Children's Fund, almost 25% of all teens worldwide, or 329 million adolescents, will reside in the East Asia and Pacific region as of January 2022. The substantial number of young people suggests that there is a considerable need for diagnostic testing for mononucleosis. Furthermore, according to a November 2022 Verywell Health article, between 25% and 50% of American children from poorer socioeconomic classes would have had EBV by the time they were 4 years old.
Furthermore, following an EBV infection, 75% of young adults will receive a mono diagnosis. Throughout the projection period, the rising cases of mononucleosis and EBV in the region are expected to drive market growth. Moreover, China’s Mononucleosis Diagnostic Market held the largest market share, and the Indian Mononucleosis Diagnostic Market was the fastest-growing market in the Asia-Pacific region.