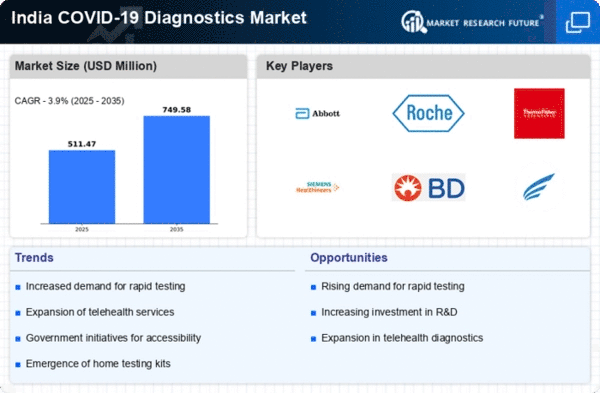
Rising Demand for Rapid Testing
The COVID-19 Diagnostics Market in India experiences a notable surge in demand for rapid testing solutions. This trend is driven by the need for quick results, particularly in high-density urban areas where timely diagnosis is crucial. The market for rapid antigen tests has expanded significantly, with a reported growth of approximately 30% in the last year alone. Healthcare facilities and laboratories are increasingly adopting these tests to enhance their diagnostic capabilities. The convenience and speed of rapid testing cater to the public's need for immediate results, thereby influencing the overall dynamics of the covid 19-diagnostics market. As the population becomes more aware of the importance of early detection, the demand for rapid testing solutions is likely to continue its upward trajectory.
Increased Government Initiatives
Government initiatives play a pivotal role in shaping the covid 19-diagnostics market in India. The Indian government has implemented various programs aimed at enhancing testing capabilities and accessibility. For instance, the introduction of free testing in public health facilities has significantly increased the number of tests conducted. Reports indicate that the number of daily tests has risen by over 50% since the implementation of these initiatives. Furthermore, the government is actively collaborating with private sectors to ensure a steady supply of diagnostic kits. These efforts not only bolster public health but also stimulate growth within the covid 19-diagnostics market, as increased testing leads to higher demand for diagnostic products and services.
Expansion of Distribution Channels
The COVID-19 Diagnostics Market in India is experiencing an expansion of distribution channels, which is crucial for enhancing accessibility to diagnostic products. The rise of e-commerce platforms and partnerships with local pharmacies have made testing kits more readily available to the public. This shift is particularly important in rural areas where access to healthcare facilities is limited. Reports suggest that online sales of diagnostic kits have increased by over 60% in the past year. As distribution channels continue to diversify, the covid 19-diagnostics market is likely to benefit from improved reach and availability, ultimately leading to higher testing rates and better public health outcomes.
Growing Public Awareness and Education
Public awareness regarding the importance of covid 19 testing is on the rise in India, significantly impacting the covid 19-diagnostics market. Educational campaigns by health authorities and NGOs have successfully informed the population about the benefits of early detection and regular testing. This increased awareness has led to a higher willingness among individuals to seek testing, contributing to a reported increase in testing rates by approximately 40% in urban areas. As more people recognize the value of diagnostic testing in controlling the spread of the virus, the demand for various testing options is expected to grow, further driving the expansion of the covid 19-diagnostics market.
Emergence of Innovative Diagnostic Technologies
The COVID-19 Diagnostics Market is witnessing a wave of innovative diagnostic technologies that enhance testing accuracy and efficiency. Technologies such as CRISPR-based diagnostics and next-generation sequencing are gaining traction in India. These advancements promise to deliver results with higher sensitivity and specificity, addressing the limitations of traditional testing methods. The market is projected to grow by approximately 25% over the next few years, driven by the adoption of these cutting-edge technologies. As healthcare providers seek to improve patient outcomes, the integration of innovative diagnostic solutions is likely to reshape the landscape of the covid 19-diagnostics market, making it more competitive and effective.