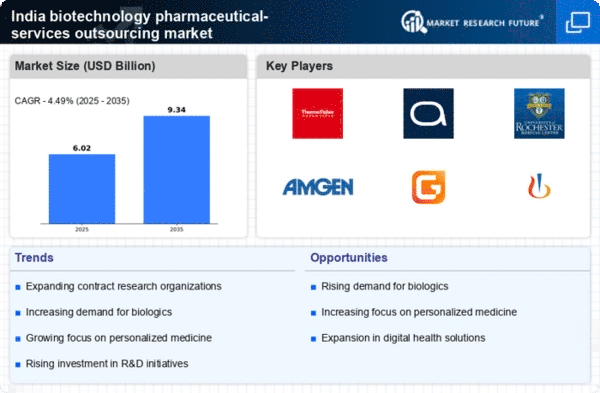
Growing Demand for Biologics
The biotechnology pharmaceutical-services-outsources market is experiencing a notable surge in demand for biologics, which are complex drugs derived from living organisms. This trend is driven by the increasing prevalence of chronic diseases and the need for targeted therapies. In India, the biologics market is projected to reach approximately $30 billion by 2025, reflecting a compound annual growth rate (CAGR) of around 15%. This growth is likely to stimulate the biotechnology pharmaceutical-services-outsources market as companies seek to develop and manufacture biologics efficiently. The rising focus on innovative treatment options is pushing pharmaceutical companies to outsource their production processes, thereby enhancing the market's dynamics.
Regulatory Support and Incentives
The Indian government is actively promoting the biotechnology sector through various regulatory support and incentives. Initiatives such as the Biotechnology Industry Research Assistance Council (BIRAC) provide funding and resources to startups and established companies alike. This supportive environment is expected to bolster the biotechnology pharmaceutical-services-outsources market, as companies are encouraged to innovate and expand their operations. The government's focus on enhancing the ease of doing business is likely to attract foreign investments, further stimulating the market. As a result, the outsourcing of pharmaceutical services is anticipated to grow, driven by favorable policies.
Increasing Focus on Cost Efficiency
Cost efficiency remains a pivotal concern for pharmaceutical companies, driving them to seek outsourcing solutions within the biotechnology pharmaceutical-services-outsources market. As companies strive to reduce operational costs, outsourcing non-core activities has become a strategic approach. In India, the cost of outsourcing is approximately 30% lower compared to developed countries, making it an attractive option for many firms. This trend is likely to continue as companies aim to allocate resources more effectively while maintaining high-quality standards. Consequently, the biotechnology pharmaceutical-services-outsources market is expected to expand as more companies recognize the financial benefits of outsourcing.
Advancements in Biotechnology Research
Innovations in biotechnology research are significantly influencing the biotechnology pharmaceutical-services-outsources market. The emergence of cutting-edge technologies such as CRISPR and gene editing is paving the way for novel therapeutic solutions. In India, research funding in biotechnology has seen an increase of over 20% in recent years, indicating a robust commitment to advancing this field. As research institutions collaborate with pharmaceutical companies, the demand for outsourcing services is likely to rise. This collaboration not only accelerates drug development timelines but also enhances the overall efficiency of the biotechnology pharmaceutical-services-outsources market.
Rising Investment in Healthcare Infrastructure
Investment in healthcare infrastructure is a critical driver for the biotechnology pharmaceutical-services-outsources market. The Indian government has allocated substantial funds to enhance healthcare facilities, which is expected to improve access to advanced medical treatments. With an estimated investment of $10 billion in healthcare infrastructure over the next few years, the biotechnology sector is poised to benefit significantly. This investment is likely to create a conducive environment for pharmaceutical companies to outsource their services, thereby fostering growth in the biotechnology pharmaceutical-services-outsources market. Enhanced infrastructure will facilitate better collaboration between research institutions and pharmaceutical companies.