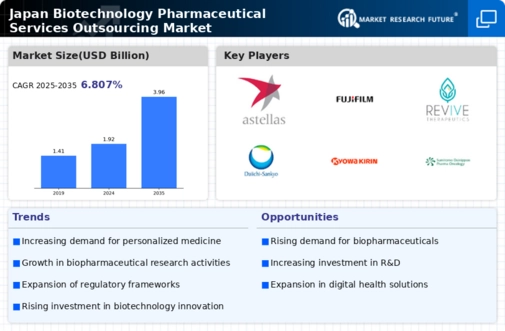
Growing Investment in Biotechnology R&D
The biotechnology pharmaceutical-services-outsources market in Japan is experiencing a surge in investment, particularly in research and development (R&D). This trend is driven by both public and private sectors, with the Japanese government allocating substantial funds to support innovative biopharmaceutical projects. In 2025, R&D expenditure in the biotechnology sector is projected to reach approximately ¥1 trillion, reflecting a growth of around 15% from the previous year. This influx of capital is likely to enhance the capabilities of local firms, enabling them to develop cutting-edge therapies and attract international partnerships. Consequently, the biotechnology pharmaceutical-services-outsources market is poised for expansion as companies leverage these investments to improve their service offerings and operational efficiencies.
Increased Focus on Personalized Medicine
The biotechnology pharmaceutical-services-outsources market is witnessing a paradigm shift towards personalized medicine, driven by advancements in genomics and biotechnology. In Japan, the demand for tailored therapies is growing, with an estimated market size of ¥500 billion projected for 2025. This shift is prompting pharmaceutical companies to invest in biotechnological solutions that cater to individual patient profiles, thereby enhancing treatment efficacy. As a result, the biotechnology pharmaceutical-services-outsources market is likely to expand, with service providers offering specialized capabilities in genetic testing and biomarker identification. This focus on personalized medicine not only improves patient outcomes but also creates new opportunities for collaboration between biotech firms and outsourcing partners.
Technological Advancements in Drug Development
The biotechnology pharmaceutical-services-outsources market is being transformed by rapid technological advancements in drug development processes. Innovations such as artificial intelligence (AI), machine learning, and high-throughput screening are streamlining research and enhancing the efficiency of clinical trials. In Japan, the adoption of these technologies is projected to increase by 20% in the next few years, enabling companies to reduce time-to-market for new drugs. This technological evolution not only improves the quality of biopharmaceutical products but also encourages collaboration between biotech firms and service providers. As a result, the biotechnology pharmaceutical-services-outsources market is likely to benefit from enhanced productivity and reduced costs associated with drug development.
Aging Population and Increased Healthcare Needs
Japan's demographic shift towards an aging population is significantly impacting the biotechnology pharmaceutical-services-outsources market. With over 28% of the population aged 65 and older, there is a growing demand for advanced medical treatments and personalized healthcare solutions. This demographic trend is likely to drive the need for biopharmaceutical innovations, particularly in areas such as oncology and chronic disease management. As healthcare providers seek to address these needs, the biotechnology pharmaceutical-services-outsources market is expected to expand, with an estimated growth rate of 10% annually. This increasing focus on tailored therapies presents opportunities for outsourcing services that can support the development and distribution of these specialized treatments.
Regulatory Support for Biopharmaceutical Innovations
The regulatory landscape in Japan is evolving to better support the biotechnology pharmaceutical-services-outsources market. Recent initiatives by the Pharmaceuticals and Medical Devices Agency (PMDA) aim to expedite the approval process for innovative therapies, particularly those addressing unmet medical needs. This regulatory flexibility is expected to foster a more conducive environment for biopharmaceutical companies, potentially increasing the number of new product launches by 25% over the next five years. As firms navigate these streamlined processes, the biotechnology pharmaceutical-services-outsources market is likely to see a rise in outsourcing activities, as companies seek specialized expertise to comply with regulatory requirements and enhance their market access.