Rising Demand for Gene Therapies
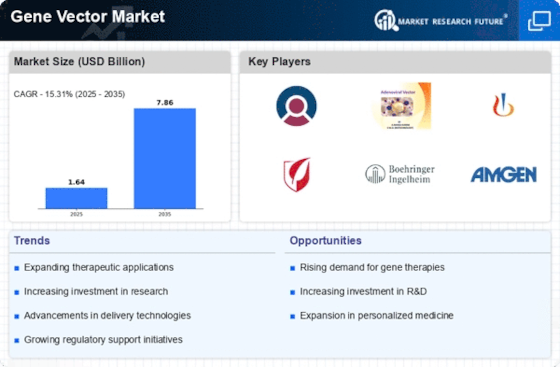
The Gene Vector Market is experiencing a notable surge in demand for gene therapies, driven by the increasing prevalence of genetic disorders and chronic diseases. As healthcare systems evolve, there is a growing recognition of the potential of gene therapies to provide long-term solutions. According to recent estimates, the gene therapy market is projected to reach approximately 10 billion USD by 2026, indicating a robust growth trajectory. This demand is further fueled by advancements in vector technologies, which enhance the efficacy and safety of gene delivery systems. Consequently, the Gene Vector Market is poised to benefit from this trend, as more pharmaceutical companies invest in developing innovative gene therapies to address unmet medical needs.
Advancements in Vector Technologies
Technological innovations in vector design and delivery systems are significantly influencing the Gene Vector Market. Recent developments in viral and non-viral vectors have improved the precision and efficiency of gene delivery, thereby enhancing therapeutic outcomes. For instance, the emergence of CRISPR-based technologies has revolutionized gene editing, allowing for more targeted interventions. The market for gene delivery systems is expected to grow at a compound annual growth rate of over 20% in the coming years, reflecting the increasing reliance on advanced vector technologies. These advancements not only improve the safety profile of gene therapies but also expand their applicability across various therapeutic areas, thereby driving growth in the Gene Vector Market.
Regulatory Support for Gene Therapy Approvals
The regulatory landscape surrounding gene therapies is evolving, with agencies providing more streamlined pathways for approval. This supportive environment is crucial for the Gene Vector Market, as it facilitates faster access to innovative therapies. Recent initiatives by regulatory bodies have aimed to expedite the review process for gene therapies, recognizing their potential to address critical health challenges. As a result, the number of approved gene therapies is on the rise, with several new products entering the market each year. This trend not only enhances the credibility of gene therapies but also encourages further investment and research within the Gene Vector Market, ultimately benefiting patients in need of advanced treatment options.
Increased Investment in Research and Development
Investment in research and development within the Gene Vector Market is witnessing a significant uptick, as stakeholders recognize the potential of gene therapies. Pharmaceutical companies, biotech firms, and academic institutions are allocating substantial resources to explore novel gene delivery methods and therapeutic applications. This trend is evidenced by the increase in clinical trials focusing on gene therapies, with over 1,000 trials currently registered worldwide. The financial backing for these initiatives is indicative of a broader commitment to advancing gene therapy technologies. As a result, the Gene Vector Market is likely to experience accelerated growth, driven by the continuous influx of innovative solutions and therapeutic options.
Growing Awareness and Acceptance of Gene Therapies
The Gene Vector Market is benefiting from a growing awareness and acceptance of gene therapies among healthcare professionals and patients. Educational initiatives and successful case studies have contributed to a more informed understanding of the benefits and risks associated with gene therapies. This shift in perception is crucial, as it encourages healthcare providers to consider gene therapies as viable treatment options. Furthermore, patient advocacy groups are playing a pivotal role in promoting awareness, leading to increased demand for gene-based treatments. As acceptance continues to rise, the Gene Vector Market is expected to expand, with more patients seeking innovative therapies for their conditions.