Increasing Funding and Investment
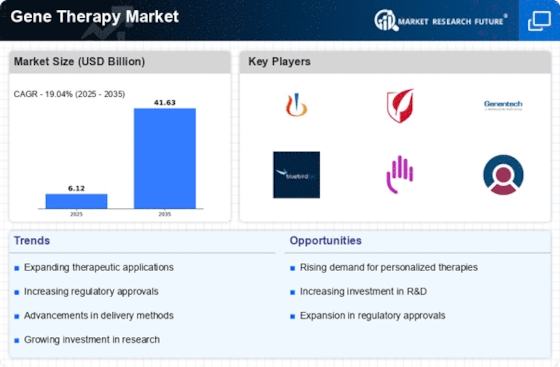
The Gene Therapy Market is experiencing a surge in funding and investment from both public and private sectors. Governments and venture capitalists are recognizing the potential of gene therapies to revolutionize treatment paradigms, leading to increased financial support for research initiatives. In recent years, funding for gene therapy projects has escalated, with billions of dollars allocated to support clinical trials and product development. This influx of capital is likely to enhance the pace of innovation within the Gene Therapy Market, facilitating the development of new therapies that address unmet medical needs. Moreover, partnerships between biotech firms and academic institutions are becoming more common, further driving collaborative efforts to advance gene therapy research and commercialization.
Growing Demand for Targeted Therapies
The shift towards personalized and targeted therapies is a notable driver for the Gene Therapy Market. Patients and healthcare providers are increasingly seeking treatments that are tailored to individual genetic profiles, which gene therapies can provide. This trend is supported by advancements in genomics and biotechnology, enabling the development of therapies that address specific genetic mutations. As of 2025, the market for targeted therapies is projected to grow substantially, reflecting a broader movement towards precision medicine. The Gene Therapy Market is well-positioned to capitalize on this demand, as it offers innovative solutions that align with the principles of personalized healthcare. Consequently, the emphasis on targeted therapies is likely to drive further investment and research in the gene therapy sector.
Rising Prevalence of Genetic Disorders
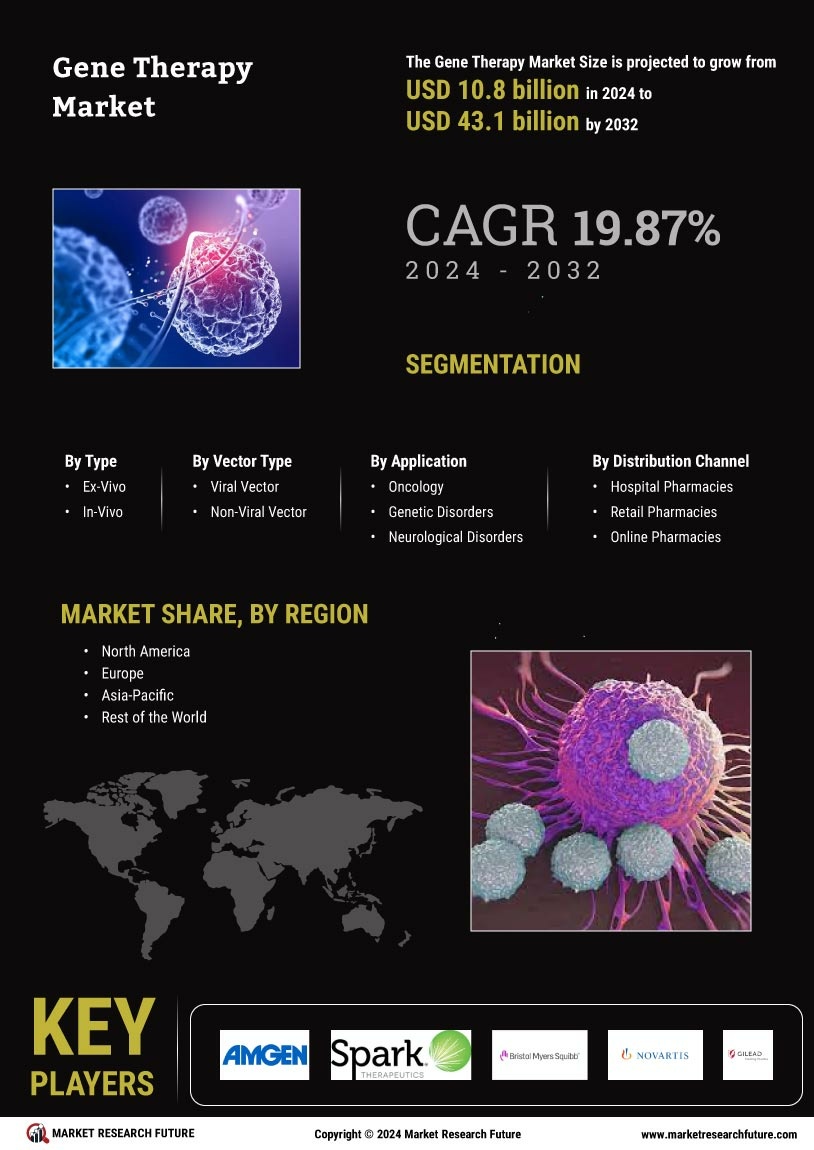
The increasing incidence of genetic disorders is a primary driver for the Gene Therapy Market. Conditions such as hemophilia, muscular dystrophy, and cystic fibrosis are becoming more prevalent, necessitating innovative treatment options. According to recent estimates, approximately 1 in 1,500 individuals are affected by hemophilia, highlighting the urgent need for effective therapies. This growing patient population is likely to propel demand for gene therapies, as traditional treatments often fall short in efficacy. The Gene Therapy Market is thus positioned to expand significantly, as healthcare providers seek advanced solutions to address these complex genetic conditions. Furthermore, the rising awareness among patients and healthcare professionals about the potential of gene therapy to provide long-term solutions is expected to further stimulate market growth.
Technological Advancements in Gene Editing
Technological innovations in gene editing techniques, such as CRISPR-Cas9 and TALENs, are transforming the Gene Therapy Market. These advancements enable precise modifications to genetic material, enhancing the efficacy and safety of gene therapies. The ability to target specific genes with high accuracy has opened new avenues for treating previously untreatable conditions. As of 2025, the market for gene editing technologies is projected to reach several billion dollars, indicating robust growth potential. This surge in technological capabilities is likely to attract significant investment, fostering further research and development in the Gene Therapy Market. Consequently, the integration of these cutting-edge technologies is expected to accelerate the approval and commercialization of novel gene therapies, thereby expanding the market landscape.
Regulatory Support and Streamlined Approval Processes
Regulatory bodies are increasingly providing support for the Gene Therapy Market by streamlining approval processes for new therapies. Initiatives aimed at expediting the review of gene therapies are being implemented, which could significantly reduce the time it takes for innovative treatments to reach the market. For instance, the introduction of fast-track designations and priority review pathways is encouraging companies to invest in gene therapy development. This regulatory environment is likely to foster a more favorable landscape for the Gene Therapy Market, as it enhances the likelihood of successful product launches. As a result, the combination of supportive regulations and a growing pipeline of gene therapies is expected to contribute to the overall expansion of the market.