Expanding Applications in Vaccines
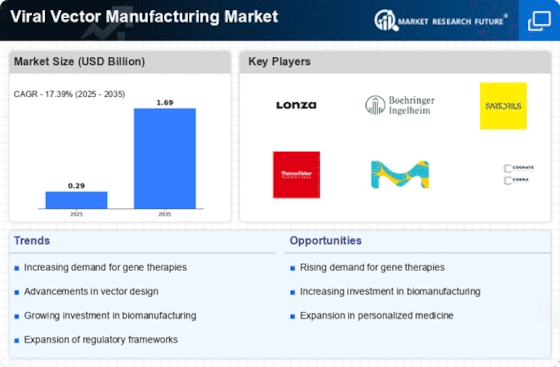
The Viral Vector Manufacturing Market is experiencing growth due to the expanding applications of viral vectors in vaccine development. With the increasing prevalence of infectious diseases, there is a pressing need for effective vaccines, and viral vectors offer a promising solution. The market for viral vector-based vaccines is anticipated to grow significantly, driven by advancements in vector design and production techniques. In 2025, the demand for such vaccines is expected to rise, as they provide a platform for rapid response to emerging pathogens. This trend underscores the importance of the Viral Vector Manufacturing Market in addressing public health challenges and enhancing vaccine accessibility.
Rising Demand for Personalized Medicine
The increasing emphasis on personalized medicine is a pivotal driver for the Viral Vector Manufacturing Market. As healthcare shifts towards tailored therapies, the need for specific viral vectors that can deliver targeted treatments becomes paramount. This trend is evidenced by the growing number of clinical trials focusing on gene therapies, which utilize viral vectors for precise delivery. In 2025, the market for personalized medicine is projected to reach substantial figures, indicating a robust demand for innovative solutions. The Viral Vector Manufacturing Market is likely to benefit from this shift, as companies strive to develop vectors that cater to individual patient profiles, enhancing treatment efficacy and safety.
Regulatory Support for Gene Therapy Products
Regulatory bodies are increasingly supportive of gene therapy products, which is a crucial driver for the Viral Vector Manufacturing Market. The establishment of clear guidelines and expedited approval processes for gene therapies has fostered an environment conducive to innovation. In recent years, several gene therapies have received regulatory approval, paving the way for more products to enter the market. This trend is likely to continue, as regulatory agencies recognize the potential of viral vectors in delivering transformative therapies. The Viral Vector Manufacturing Market stands to gain from this supportive regulatory landscape, facilitating the development and commercialization of new gene therapies.
Technological Innovations in Vector Production
Technological innovations in vector production are significantly influencing the Viral Vector Manufacturing Market. Advances in manufacturing processes, such as the use of suspension cell cultures and improved purification techniques, are enhancing the efficiency and scalability of viral vector production. These innovations are crucial as the demand for viral vectors continues to rise, particularly in gene therapy and vaccine development. In 2025, the market is expected to witness a surge in production capabilities, driven by these technological advancements. The Viral Vector Manufacturing Market is poised to leverage these innovations to meet the growing needs of biopharmaceutical companies and research institutions.
Increased Funding for Biopharmaceutical Research
The surge in funding for biopharmaceutical research is a significant driver for the Viral Vector Manufacturing Market. As governments and private investors allocate more resources towards innovative therapies, the demand for viral vectors is likely to increase. This funding supports research initiatives that explore the potential of viral vectors in treating various diseases, including genetic disorders and cancers. In 2025, the biopharmaceutical sector is projected to receive substantial investments, which will, in turn, bolster the Viral Vector Manufacturing Market. This influx of capital is expected to accelerate the development of new viral vector technologies and expand their applications in therapeutic areas.