Gene Therapy Market Summary
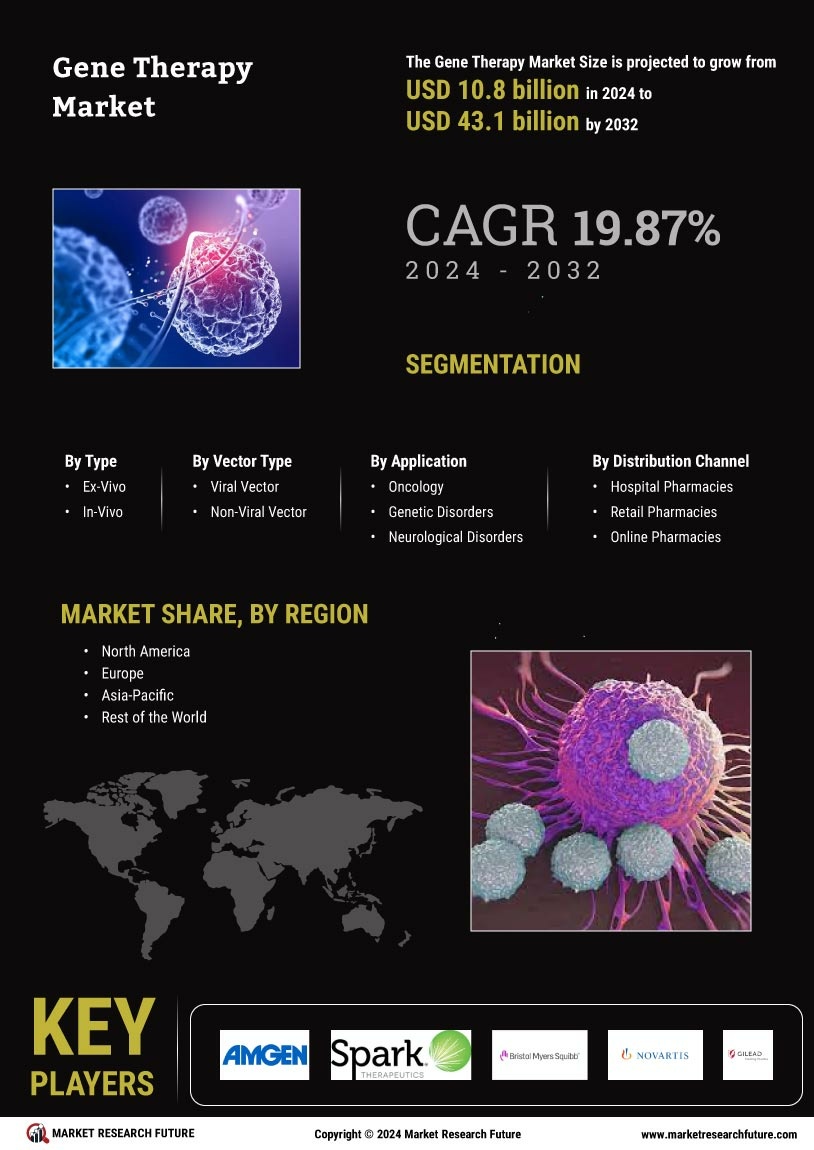
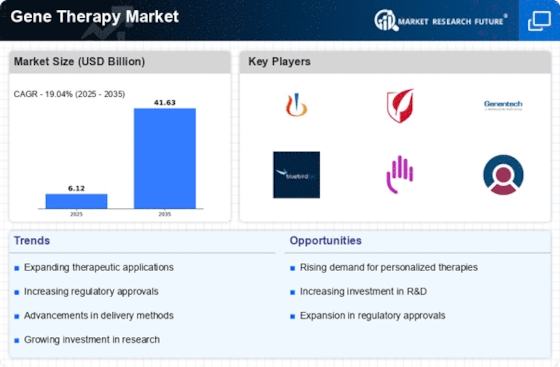
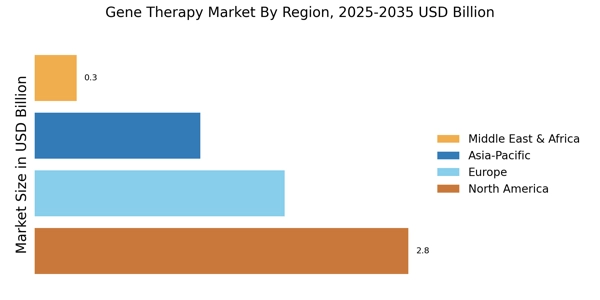
According to MRFR analysis, the Gene Therapy Market Size was valued at USD 6.12 Billion in 2024. The market is projected to grow from USD 7.285 Billion in 2025 to USD 41.63 Billion by 2035, registering a CAGR of 19.04% during the forecast period (2025–2035). North America dominated the market with the largest revenue share of 45.75% in 2024.
The Gene Therapy Market is expanding rapidly due to increasing prevalence of genetic disorders, rising investment in biotechnology research, and growing approvals of advanced therapies. Key trends include advancements in CRISPR and viral vector technologies, expanding clinical trials, and strong funding for precision medicine, driving innovation and commercialization of gene-based treatments globally.
Key Market Trends & Highlights
The Gene Therapy Market is poised for substantial growth driven by technological advancements and increasing demand for personalized medicine.
- North America remains the largest market for gene therapy, driven by robust investment in research and development.
- The Asia-Pacific region is emerging as the fastest-growing market, fueled by increasing healthcare expenditure and innovation.
- Viral vector therapies dominate the market, while non-viral vector approaches are rapidly gaining traction due to their potential for safer applications.
- Rising prevalence of genetic disorders and regulatory support for streamlined approval processes are key drivers propelling market expansion.
Market Size & Forecast
| 2024 Market Size | 6.12 (USD Billion) |
| 2035 Market Size | 41.63 (USD Billion) |
| CAGR (2025 - 2035) | 19.04% |
Major Players
Novartis (CH), Gilead Sciences (US), Spark Therapeutics (US), Bluebird Bio (US), Bristol-Myers Squibb (US), Sangamo Therapeutics (US), CRISPR Therapeutics (CH), AstraZeneca (GB), Roche (CH)