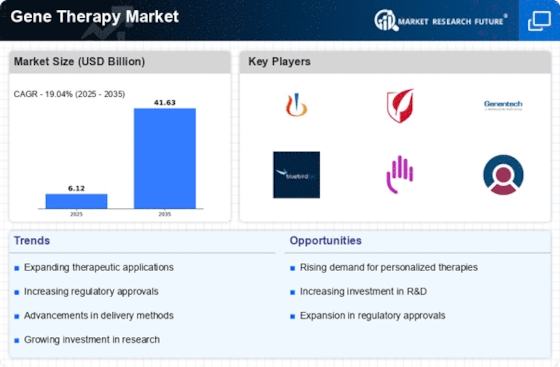
Market Trends
Key Emerging Trends in the Gene Therapy Market
Breakthroughs in genetic research, innovation, and an increasing understanding of gene-based therapies are transforming the Gene Therapy Market. Rare genetic illnesses are becoming gene therapy targets. Gene treatments for uncommon ailments are being developed as disease-causing genetic abnormalities are discovered. This move toward more specific and precise therapies gives hope to people with untreatable illnesses.
Oncology gene treatments are growing in the market. Researchers are investigating gene-based methods to target cancer cells while protecting healthy organs. Chimeric Antigen Receptor (CAR) T-cell treatments, a kind of gene therapy, have showed promise in hematological malignancies, opening the door for cancer therapeutic improvements. This trend shows gene therapy's dramatic influence on cancer. Ex vivo gene therapy allows precise genetic alterations by changing patient cells outside the body and reintroducing them. This paradigm change in gene therapy administration allows for more precise and tailored treatments.
Gene treatments for monogenic illnesses are growing in popularity. Understanding the genetics of single-gene illnesses has led to gene-correction and replacement treatments. Curative techniques attempt to treat genetic problems at their source and give long-term benefits.
Technological advances in gene delivery technologies shape the Gene Therapy Market. Researchers are investigating AAVs and lentiviruses to improve gene delivery efficiency and safety. These technologies attempt to bypass immune reactions and off-target consequences, making gene treatments more successful and applicable. Optimizing delivery systems for gene-based therapeutic translation is highlighted by this trend.
Interest in RNA-based gene treatments also affects the market. RNA therapeutics like mRNA and siRNA may be more selective and adaptable. This trend shows the adaptability of RNA-based gene expression modulation and opens new treatment pathways for a variety of hereditary disorders.
Gene therapy research and development depend on biopharmaceutical, academic, and healthcare relationships. Joint clinical trials, data exchange, and infrastructure development accelerate gene-based therapeutics. This collaboration shows gene therapy's multidisciplinary character and common dedication to genetic medicine's promise.
The market is also paying more attention to gene therapy price and regulation. Regulators and reimbursement models must address gene-based therapeutic development, manufacturing, and price difficulties. This increase reflects changing healthcare policies and the need for sustained gene therapy availability.

















Leave a Comment