Adoption of Cloud-Based Solutions
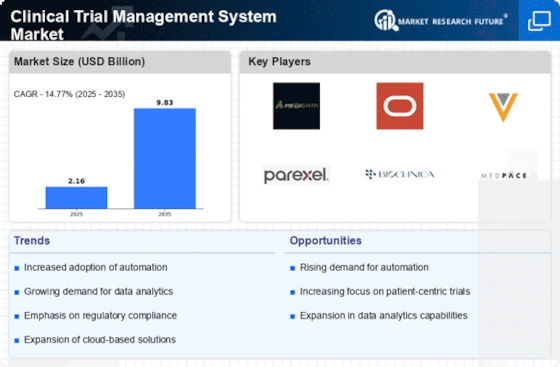
The shift towards cloud-based solutions is transforming the Clinical Trial Management System Market. Organizations are increasingly recognizing the advantages of cloud technology, including scalability, cost-effectiveness, and enhanced collaboration. This trend is reflected in the growing number of companies opting for cloud-based clinical trial management systems, which allow for real-time data access and improved communication among stakeholders. The market is anticipated to expand significantly as more organizations transition to these solutions, with estimates suggesting a growth rate of approximately 15% over the next few years. The flexibility offered by cloud-based systems enables organizations to adapt to changing trial requirements swiftly, thereby enhancing overall operational efficiency. As the industry continues to evolve, the adoption of cloud technology is likely to play a pivotal role in shaping the future of the Clinical Trial Management System Market.
Focus on Patient-Centric Approaches
The Clinical Trial Management System Market is increasingly influenced by a focus on patient-centric approaches. As stakeholders recognize the importance of patient engagement in clinical trials, management systems are evolving to incorporate features that enhance the patient experience. This shift is evident in the development of systems that facilitate better communication between researchers and participants, as well as tools that allow for remote monitoring and data collection. The industry is projected to grow at a rate of around 11% annually, driven by the need for more inclusive and accessible trial designs. By prioritizing patient needs, organizations can improve recruitment and retention rates, ultimately leading to more successful trial outcomes. This emphasis on patient-centricity is likely to continue shaping the Clinical Trial Management System Market, as organizations strive to align their operations with the evolving expectations of patients.
Regulatory Compliance and Standardization
Regulatory compliance remains a critical driver within the Clinical Trial Management System Market. As regulatory bodies impose stringent guidelines to ensure patient safety and data integrity, organizations are compelled to adopt comprehensive management systems that facilitate adherence to these regulations. The industry is witnessing a notable increase in the implementation of systems designed to automate compliance processes, thereby minimizing the risk of non-compliance. This trend is particularly evident in regions where regulatory frameworks are evolving rapidly. The Clinical Trial Management System Market is expected to benefit from this shift, as organizations prioritize systems that not only streamline operations but also ensure compliance with local and international regulations. Consequently, the demand for sophisticated management solutions is likely to rise, reinforcing the importance of regulatory compliance in shaping market dynamics.
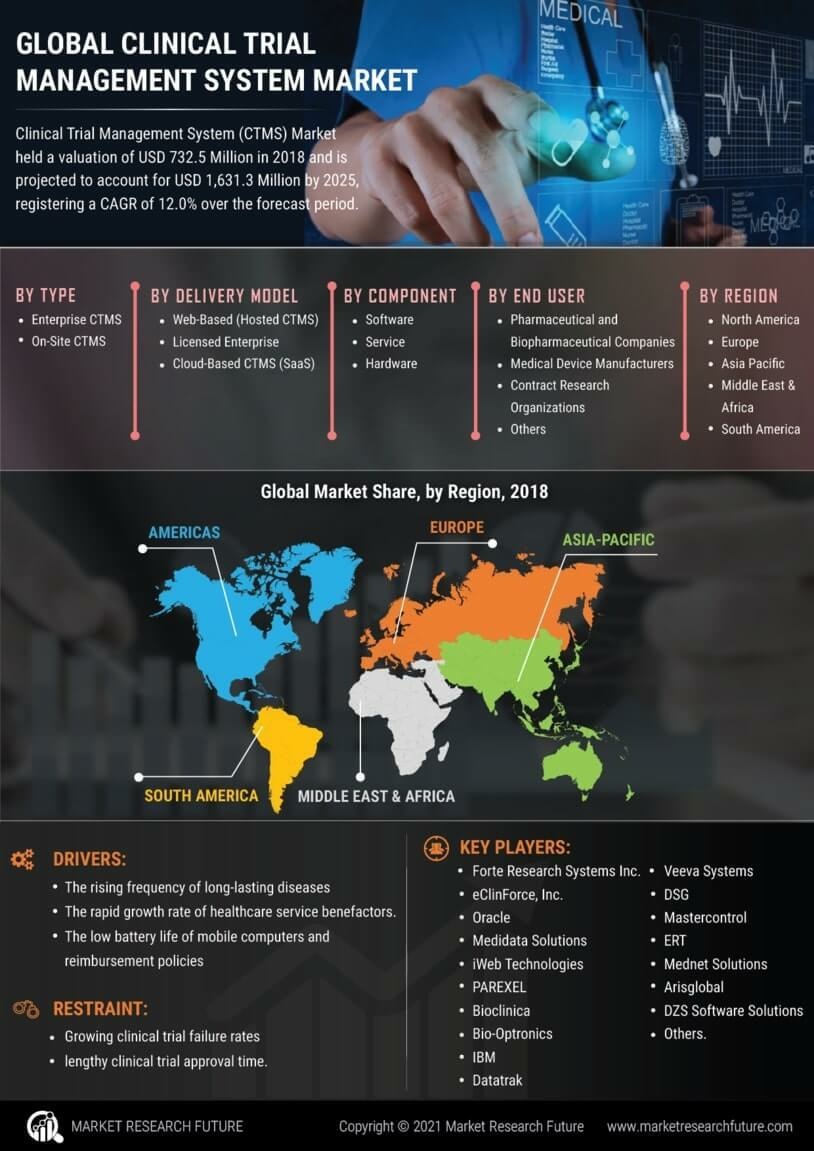
Rising Demand for Efficient Clinical Trials
The Clinical Trial Management System Market is experiencing a surge in demand for efficient clinical trials. As pharmaceutical companies and research organizations strive to expedite drug development processes, the need for streamlined operations becomes paramount. The industry is projected to grow at a compound annual growth rate of approximately 12% over the next five years. This growth is driven by the increasing complexity of clinical trials, necessitating robust management systems to handle diverse data sources and regulatory requirements. Furthermore, the integration of advanced technologies within these systems enhances operational efficiency, thereby attracting more stakeholders to invest in Clinical Trial Management Systems. The focus on reducing time-to-market for new therapies further propels this demand, indicating a strong trajectory for the Clinical Trial Management System Market.
Increased Investment in Research and Development
Investment in research and development (R&D) is a significant driver for the Clinical Trial Management System Market. As biopharmaceutical companies allocate substantial resources to R&D, the need for effective management systems becomes increasingly apparent. The industry is projected to witness a growth rate of around 10% annually, fueled by the rising number of clinical trials initiated worldwide. This trend is indicative of a broader commitment to innovation and the development of new therapies. Moreover, the growing emphasis on personalized medicine and targeted therapies necessitates advanced management systems capable of handling complex trial designs and diverse patient populations. As organizations seek to optimize their R&D processes, the Clinical Trial Management System Market stands to gain from heightened investment in innovative management solutions.