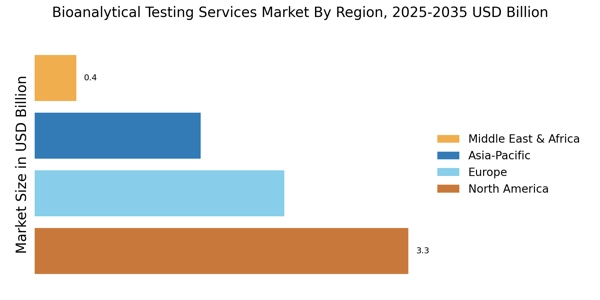
The Bioanalytical Testing Services Market is currently experiencing a dynamic evolution, driven by the increasing demand for accurate and reliable testing methods in the pharmaceutical and biotechnology sectors. As drug development processes become more complex, the need for bioanalytical testing services has intensified.
Bioanalytical service encompasses a wide range, such pharmacokinetics, bioavailability studies, and biomarker analysis, which are essential for ensuring the safety and efficacy of new therapeutics.
Furthermore, advancements in technology, such as high-throughput screening and mass spectrometry, are enhancing the capabilities of testing laboratories, thereby expanding the scope of bioanalytical services available to clients. Advanced bioanalytical testing is now essential for validating the safety and efficacy of next-generation antibody-drug conjugates.
In addition to technological advancements, regulatory requirements are becoming more stringent, necessitating the adoption of robust testing protocols. This trend is likely to drive the growth of the Bioanalytical Testing Services Market as companies seek to comply with evolving guidelines.
Moreover, the increasing focus on personalized medicine and the development of biologics are expected to further propel the demand for specialized bioanalytical services. The bioanalytical services market is expanding as emerging biotech startups rely on external partners for their entire analytical needs. As the market continues to mature, collaboration between testing service providers and pharmaceutical companies may become more prevalent, fostering innovation and improving overall service delivery.
Overall, the Bioanalytical Testing Services Market appears poised for sustained growth, reflecting the critical role it plays in the drug development landscape.
Technological Advancements
The Bioanalytical Testing Services Market is witnessing a surge in technological innovations that enhance testing accuracy and efficiency. New methodologies, such as advanced mass spectrometry and high-throughput screening, are being integrated into laboratory practices. These advancements not only streamline processes but also enable the analysis of complex biological samples, thereby expanding the range of services offered.
Regulatory Compliance
As regulatory bodies impose stricter guidelines, the Bioanalytical Testing Services Market is adapting to meet these requirements. Companies are increasingly investing in quality assurance and validation processes to ensure compliance with international standards. Each specialized bioanalytical service must comply with strict GLP standards to ensure that data is acceptable for international regulatory submissions. This trend underscores the importance of reliable testing services in maintaining product integrity and safety.
Focus on Personalized Medicine
The growing emphasis on personalized medicine is reshaping the Bioanalytical Testing Services Market. As treatments become more tailored to individual patient profiles, the demand for specialized bioanalytical services is likely to increase. This shift necessitates the development of innovative bio analytical services methods that can accurately assess the efficacy of targeted therapies.